Clinical efficacy of icotinib in patients with advanced nonsquamous non-small cell lung cancer with unknown EGFR mutation status that failed to respond to second-line chemotherapy
Introduction
Non-small cell lung cancer (NSCLC), accounting for 85% of all lung cancer cases, is the most common and most aggressive malignant lung cancer in humans, and more than 50% cases among them are nonsquamous NSCLC (1-3). In addition, 60–70% patients have been advanced when diagnosed. Most of the patients with NSCLC that is advanced or recurrent after surgery have lost the opportunities for radical surgery or radiotherapy. As the driver gene status is unknown, the preferred standard treatments are the two kinds of third generation platinum-based chemotherapy. Patients with first-line treatment failure or disease progression could select the second-line chemotherapy without cross-resistance. However, research (ECOG1594) has proved that disease time to progression (TTP) is less than 4.2 months upon treatment with any of the third generation chemotherapy treatments. For patients with nonsquamous NSCLC who fail to respond to second-line chemotherapy, there is no more effective chemotherapeutic cytotoxic drug available, so biological targeting treatment is the main option. However, current clinical treatment is based on the guidance of clear driving gene mutations. Now, we are facing a tricky situation of clinical status in which the physical state is poor after chemotherapy; organ function recesses and organizational biopsy compliance is poor, which leads to a large number of patients with unknown driving gene mutation failing to respond to second-line chemotherapy.
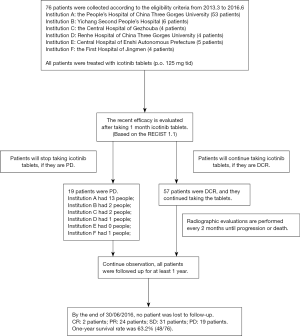
We performed targeted therapy with the first generation EGFR-TKI, Icotinib, on 76 advanced nonsquamous NSCLC patients who failed to respond to second-line chemotherapy or whose disease progressed and developed systematically during March 2013 to June 2016 to detect the curative effects, side effects and the 1-year survival rate.
Methods
The flow chart about the patient selection was shown as the supplementary material (Figure S1). The eligibility criteria were as follows: the ages of males and females were between 18 and 75 years; advanced nonsquamous NSCLC was diagnosed by histopathology or cytology; the first diagnosis was not available for genetic detection of tissue samples and EGFR mutation status was unknown; patients failed to respond to second-line chemotherapy. Treatment failure means that the disease relapsed, or metastasis occurred during or after the treatment or intolerable toxicity occurs. The second-line chemotherapy refers to the two kinds of platinum-based chemotherapy of the third generation (PC, GC or TC). The treatment time is greater than or equal to 2 cycles with the use of one or more kind of chemotherapy drug for a longer time; Eastern Cooperative Oncology Group (ECOG) body condition score (performance status, PS) was between 1–2 points; according to the Response Evaluation Criteria in Solid Tumors (RECIST) standard, there was at least one measurable lesion; indicators of chemotherapy of blood, liver, kidney and heart functions were normal. White blood cells ≥4.0×109/L, neutrophils ≥2.0×109/L, platelet ≥60×109/L, hemoglobin ≥80 g/L, serum bilirubin below the maximum amount of 1.5 times, and both alanine aminotransferase (ALT) and aspartate transaminase (AST) were 1.5 times lower than the normal maximum; lifetime was expected to be more than 1 month; patients could understand the significance of this study and signed informed consent.
Patients and tumor characteristics
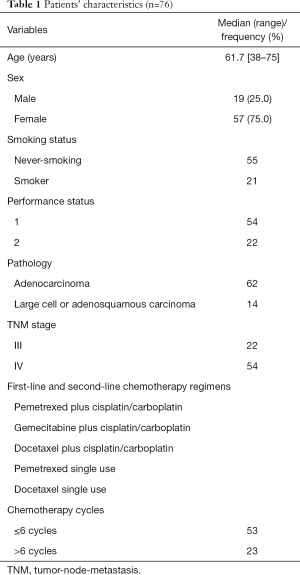
As shown in Table 1, 76 patients with advanced nonsquamous NSCLC who had failed with the second-line chemotherapy were investigated from March 2013 to June 2016 at seven hospitals of Hubei province, China (The First College of Clinical Medical Science of China Three Gorges University, the People’s Hospital of China Three Gorges University, Yichang Second People’s Hospital, the Central Hospital of Gezhouba, Renhe Hospital of China Three Gorges University, Central Hospital of Enshi Autonomous Prefecture and the First Hospital of Jingmen), including 19 males and 57 females aged between 38 and 75 years. Among all patients, 21 were ever-smokers (smoking more than 6 months) and 55 cases were never-smokers (smoking less than 6 months). All cases were diagnosed by histology or cytology. Among them, 49 cases were diagnosed by fiberoptic bronchoscopic biopsy or bronchoscopy brush, and another 27 cases by lymph node biopsy or percutaneous lung puncture. Patients have a variety of tumor types; 62 cases were lung adenocarcinoma, 10 cases were large cell carcinoma and 4 cases were adenosquamous carcinoma. When first diagnosed, EGFR mutation detection was not executed due to lack of tissue samples. Twenty-seven patients with a primary lesion or mediastinum lesion received radiotherapy.
Full table
All patients failed to respond to second-line chemotherapy with two kinds platinum-based chemotherapy of the third generation. They were also physically examined by enhanced CT of chest and abdomen, brain magnetic resonance imaging (MRI), and whole-body radionuclide scan. Few of them were examined by positron emission tomography-computed tomography (PET/CT). All patients were divided into groups by the international tumor-node-metastasis (TNM) staging system (AJCC Cancer Staging Manual, 7th Edition, Lung cancer): 5 cases were stage III A, 17 cases were stage III B and another 54 cases were stage IV. In addition, there was at least one measurable lesion at the metastatic sites, including lung, brain, liver, bone and adrenal gland, and 23 cases with brain metastases, which received intensity modulated radiation therapy (IMRT). Additionally, 54 cases received 1 point, and 22 cases received 2 points, by the performance status (PS) score, and the expected survival was more than 1 month. All patients were treated without an EGFR-TKI before admission, received icotinib treatment voluntarily and signed informed consent. The study was approved by the Ethics Committee of all seven hospitals.
Treatment regimens
All patients were treated with 125 mg icotinib tablets (Zhejiang Beida Pharmaceutical Co. Ltd. production) orally three times per day. The remaining adjuvant therapy, such as pain management, nutritional support, brain radiation and phosphate, were selected according to the disease conditions.
Efficacy evaluation
The recent efficacy was evaluated according to the RECIST by the World Health Organization (WHO) and was divided into four parts: complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Response rate (RR) = (CR + PR)/total disease*100%, disease control rate (DCR) = (CR + PR + SD)/total disease*100%. The first evaluation was performed at almost 1 month after chemotherapy and included radiographic evaluation and clinical benefits. If the radiographic features showed a trend of increased lesions or patients did not experience any clinical benefits and there was no rash, diarrhea or other toxic side effects, the treatment indicated it to be ineffective and the drug was withdrawn; if not, drugs would be continued and evaluated every 2 months by radiograph to detect the number and size of lesions until disease progression or the emergence of intolerable toxicity. The result is the best overall response, which is the best response recorded from the start of the study treatment until the end of treatment taking into account any requirement for confirmation.
The criteria of clinical benefit rate (CBR) was performed according to the standards established by Burris et al. (4), including the amount of pain medications or pain relief, as well as changes in the Karnofsky performance score (KPS score) and body weight. Patients were evaluated using the KPS score, and the body weight was measured weekly (duration ≥4 weeks). In addition, a reduction in pain medications by more than 50%, KPS score reduced by more than 20%, or body weight increased by more than 7% were identified as clinical benefits as long as one of the three criteria was sustained for more than 4 weeks.
Survival follow-up
Follow-up was calculated from the day of diagnosis to the date of the event or last follow-up visit, and the survival time was defined as the time between the beginning of the investigation and the death time or the time of the latest follow-up. The radiographic evaluation of follow-up was performed by chest CT. All patients were followed-up every month for the first 1 year, every 3 months for the next 2 years and then annually. All patients were followed up for at least 1 year through correspondence and outpatient department visits, and none of the patient were lost. Progression-free survival (PFS) was defined as the time from the beginning of the investigation to the tumor progression or the patient’s death.
Statistical analysis
All statistical analyses were performed using SPSS10.0 (SPSS Inc., Chicago, IL, USA). The count data were compared by χ2 tests. Survival rates were calculated using the Kaplan-Meier method, and differences were compared by using the log-rank test. A two-tailed P value less than 0.05 was considered statistically significant.
Results
Short-term outcome
Short-term efficacy of targeted therapy was evaluated based on the RECIST 1.1. Among the 76 patients, 2 patients were completely remised, 24 patients were partially relieved, 31 patients were at stable phase, and 19 patients had disease progression. The overall response rate (ORR) was 34.2% (26/76), and the DCR was 75.0% (57/76). Additionally, gender and smoking history were correlated to the DCR (P=0.026, Table 2). The symptoms of 54 patients improved significantly, which manifested as alleviating a cough or asthma and pain-relieving. Appetite, physical strength and the quality of life were further improved, and the CBR was 80.2% (61/76).

Full table
Survival analysis
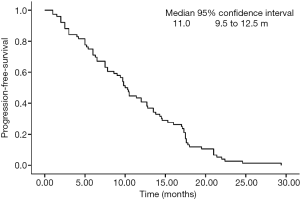
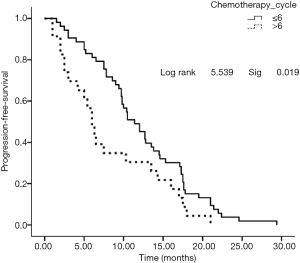
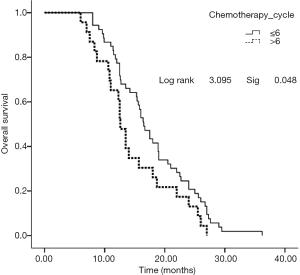
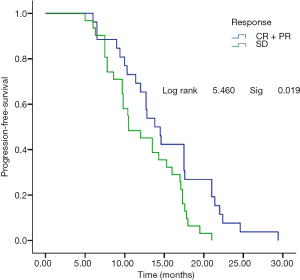
No patient was lost to follow-up before the deadline of Jun 30th, 2016. Twenty-eight cases died due to PD or systemic failure. The median PFS was 11.0 months (Figure 1), the overall survival (OS) period was 16.9 months (Figure 2), and the 1-year survival rate was 63.2% (48/76). Subgroup analysis was performed based on the course of treatment with first/second-line chemotherapy ≤6 or >6 cycles; PFS and OS were better in the former (P<0.05, Figures 3,4). Furthermore, for the subgroup analysis depending on the total effective rate (ORR) and the DCR, both PFS and OS were shown in Figures 5,6.

Adverse reactions
The main adverse reactions to Icotinib were rash and diarrhea. The incidence of rash in our investigation was 76.7% (56/76), and the incidence of diarrhea was 36.8% (28/76). Among them, 51 cases were grade I–II. Only one patient was converted to icotinib once every 2 days due to more severe diarrhea and that patient eventually achieved PR and exceeded median survival.
Conclusions
Lung cancer is one of the world’s most aggressive and deadly diseases. Moreover, nonsquamous NSCLC accounts for the vast majority of lung cancer. Once diagnosed, patients have often entered the advanced stage and lost the opportunity for radical surgery. Standard chemotherapy is still the preferred treatment for advanced nonsquamous NSCLC with unknown genetic status. After clinical or radiographic progression after first-line chemotherapy treatment, patients with a PS score of 1–2 should be considered for second-line therapy, including alimta and taxotere. However, it is still therapeutically difficulty for the patients with failure to respond to second-line chemotherapy.
EGFR mutations are widely accepted as a marker for the treatment of advanced NSCLC. However, in clinical practice, detection of EGFR mutation is confronted with many obstacles. For example, specimens from the first diagnosis can be exhausted, and there can be a lack of reacquisition because of the disease advancement, tumor heterogeneity, DNA deficiency or detection failure. Even in prospective clinical studies such as IPASS, FLEX, the ultimately effective detection rate based on tissues only varies between 30% and 40% (5,6), and the rate may be much lower in practice than in the lab. Furthermore, TAILOR and IPASS found 23% and 64.1% of patients, respectively, who were reluctant to receive EGFR gene detection (5,7). In the ICOGEN study, only 38% of the tissue samples were available (8). In our clinical practice, only approximately 45% of EGFR mutations were detected by histology, and this rate was much lower in the primary hospital. It will, therefore, be a difficult challenge to treat patients with unknown EGFR mutation status in nonsquamous NSCLC.
According to the NCCN, ESMO and Chinese guidelines, patients with unknown EGFR mutations or wild-type EGFR and PS score 0–3 who have not been previously treated with EGFR-TKIs may be considered for targeted therapy. In theory, these patients should make systematic analysis for EGFR or other related gene mutations before targeted drugs are used. The clinical reality is that we conducted with Icotinib in patients with nonsquamous NSCLC according to histology that failed to respond to second-line chemotherapy. Our results showed that RR was 34.2%, DCR was 75%, PFS was 11.2 months, and the CBR was 80.2%, all of which has reached the main points of the primary observation. Furthermore, the median OS reached 17.5 months, the 1-year OS was 63.2%, and the main adverse effects were rash and diarrhea of grade I–II, which are small, well-tolerated side effects, consistent with the ICOGEN study (8). This was amazing for advanced patients that failed to respond to second-line chemotherapy. DCR was more advanced, especially in nonsmoking or less smoking female adenocarcinoma patients (P<0.05). Previous research has demonstrated that advanced NSCLC patients with EGFR mutations were almost 10–20%, and more than 50% were positive for adenocarcinoma, Asian, nonsmoking or female patients (9). Additionally, IGNITE had also confirmed that the EGFR gene mutation rate was 49.2% in adenocarcinoma of Asia-Pacific region, which was consistent with that of PIONEER. The rate of EGFR mutation in non-adenocarcinoma patients in the Asia-Pacific region was also up to 14.1%. These findings contributed to rational explanations to the abovementioned effects.
In recent years, many phase III clinical studies [such as IPASS (5), WJTOG3405 (10), NE-JGSG (11), OPTIMAL (12), First-SIGNAL (13), EURTAC (14) and LUX-Lung3 (15)] indicated that EGFR mutation-positive patients treated with TKI as first-line treatment showed a better advantage in the PFS and quality of life compared with standard chemotherapy, while EGFR-TKI was not recommended as a first-line chemotherapy for wile-type or EGFR mutation-unknown patients.
INFORM (16) and KCSG-LU08-01 (17) have shown that treatment with EGFR-TKIs in progressing NSCLC has satisfactory effects as both second-line chemotherapy and maintenance therapy. Fiala et al. (18) have demonstrated that both PFS and OS were significantly higher when the second-line chemotherapy pemetrexed was used first and then combined with targeted chemotherapy in progressed EGFR wild-type lung adenocarcinoma. Recently, a retrospective trial performed by Bronte et al. (19) discovered TKI monotherapy for advanced NSCLC with wild-type or unknown EGFR status trended to a benefit in OS. Our results showed a higher DCR and 1-year OS in nonsquamous NSCLC among females or nonsmokers by using Icotinib, and all groups had nice clinical tolerability. Therefore, it was a recommended strategy for use in clinics.
It was worth noting that, by subgroup analysis, patients with ≤6 cycles of preoperative chemotherapy had significantly better PFS and OS than patients with >6 cycles of chemotherapy did, suggesting that prechemotherapy may affect the efficacy of late EGFR-TKI. The mechanism is worth further investigation, which may be the high heterogeneity of tumors, with EGFR-mutated cells being more sensitive to chemotherapy and tumor cells with wild type EGFR tending to survive chemotherapy, leading to a shorter PFS and OS by using targeted drugs (20).
Another noteworthy phenomenon was that there was a good agreement between the CBR and DCR in all cases (80.2% and 75.0%, respectively). In the subgroup analysis based on DCR and ORR, 26 cases of ORR compared with 31 cases with DCR. PFS in the former was significantly superior to that the latter (P=0.019), while there was no significant difference in OS (P=0.063), which indicated that PFS was not converted to survival benefits and promoted the limits of clinical curative effects of EGFR-TKIs based upon RESIST standards, which may be related to the unique mechanisms of EGFR-TKIs. Therefore, PFS cannot objectively reflect the long-term survival of patients from the imaging evaluation of lesions, and the CBR or DCR may be more objective measures of the efficacy of Icotinib (21,22).
Acknowledgements
Funding: This study was funded by Natural Science Foundation of Hubei Province, China (grant number 2014CFB312); Key supporting project of scientific research project of Health and Family Planning Commission of Hubei Province, China (grant number WJ2017Z026).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J Thorac Oncol 2008;3:819-31. [Crossref] [PubMed]
- Langer CJ, Besse B, Gualberto A, et al. The evolving role of histology in the management of advanced non-small-cell lung cancer. J Clin Oncol 2010;28:5311-20. [Crossref] [PubMed]
- Wakelee H, Zvirbule Z, De Braud F, et al. Efficacy and Safety of Onartuzumab in Combination With First-Line Bevacizumab- or Pemetrexed-Based Chemotherapy Regimens in Advanced Non-Squamous Non-Small-Cell Lung Cancer. Clin Lung Cancer 2017;18:50-9. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Pirker R, Pereira JR, Szczesna A, et al. Prognostic factors in patients with advanced non-small cell lung cancer: Data from the phase III FLEX study. Lung Cancer 2012;77:376-82. [Crossref] [PubMed]
- Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981-8. [Crossref] [PubMed]
- Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol 2013;14:953-61. [Crossref] [PubMed]
- Soria JC, Mok TS, Cappuzzo F, et al. EGFR-mutated oncogene-addicted non-small cell lung cancer: current trends and future prospects. Cancer Treat Rev 2012;38:416-30. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Micheu MM, Dorobantu M. Fifteen years of bone marrow mononuclear cell therapy in acute myocardial infarction. World J Stem Cells 2017;9:68-76. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3342-50. [Crossref] [PubMed]
- Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 2012;13:466-75. [Crossref] [PubMed]
- Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer 2012;118:6234-42. [Crossref] [PubMed]
- Fiala O, Pesek M, Finek J, et al. Sequential treatment of advanced-stage lung adenocarcinoma harboring wild-type EGFR gene: second-line pemetrexed followed by third-line erlotinib versus the reverse sequence. Anticancer Res 2013;33:3397-402. [PubMed]
- Bronte G, Franchina T, Alu M, et al. The comparison of outcomes from tyrosine kinase inhibitor monotherapy in second- or third-line for advanced non-small-cell lung cancer patients with wild-type or unknown EGFR status. Oncotarget 2016;7:35803-12. [Crossref] [PubMed]
- Chang IS, Jiang SS, Yang JC, et al. Genetic Modifiers of Progression-free Survival in Never-smoking Lung Adenocarcinoma Patients Treated with First-line TKIs. Am J Respir Crit Care Med 2017;195:663-73. [Crossref] [PubMed]
- Patil V, Noronha V, Joshi A, et al. Is There a Limitation of RECIST Criteria in Prediction of Pathological Response, in Head and Neck Cancers, to Postinduction Chemotherapy? ISRN Oncol 2013;2013. [Crossref] [PubMed]
- Levine ZH, Galloway BR, Peskin AP, et al. Tumor volume measurement errors of RECIST studied with ellipsoids. Med Phys 2011;38:2552-7. [Crossref] [PubMed]