
Cholesterol homeostasis, macrophage malfunction and age-related macular degeneration
Introduction
Age related macular degeneration (AMD) is the leading cause of blindness in the elderly in developed countries (1,2). Due to the growing aging population and increased life expectancy, the number of AMD cases is expected to be doubled by 2020 (3). The early stage of AMD is characterized by the deposition of extracellular materials beneath (basal laminar and basal linear deposition and drusen) or above (pseudo-drusen) the retinal pigment epithelium (RPE). The disease can advance into two late stages, “dry” and “wet”. Dry AMD refers to the geographic atrophy of the macula; whereas wet AMD is the growth of abnormal blood vessels into the macula, also known as neovascular AMD (nAMD). Although nAMD is treated by intravitreal injection of vascular endothelial growth factor inhibitors (e.g., anti-VEGF antibodies), the treatment is not curative, and patients eventually lose their central vision due to the development of geographic atrophy or macular fibrosis (4). Dry AMD is more common than the wet form and there is currently no treatment for it. AMD remains to be a major medical and socioeconomic challenge worldwide. Therefore, there is an urgent need for better therapies or prevention, and further understanding the underlying mechanisms of the disease is essential to achieve this.
Macrophage dysfunction in AMD
Macrophage dysfunction plays an important role in the pathogenesis of AMD (5,6). Macrophages may contribute to the development of AMD at multiple levels. When the macula suffers from oxidative insults, microglia and macrophages may migrate to the subretinal space (7), the junction between photoreceptor and RPE cells (Figure 1), with the intention to clean debris and maintain homeostasis, a phenomenon known as para-inflammation (8,9). Choroidal macrophages may also infiltrate into the sub-RPE space (2,10) (Figure 1). Once they phagocytize debris, they are removed from the subretinal space and sub-RPE space, and impaired removal of these cells causes subretinal inflammation and photoreceptor degeneration (6). If subretinal phagocytes are unable to remove debris, particularly when the RPE function is compromised and waste materials cannot be digested or transported by RPE to the choroid, subretinal drusenoid deposits (also known as pseudo-drusen) may occur.
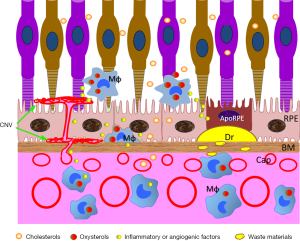
Choroidal macrophages are important scavenger cells responsible for the removal of waste materials and debris from the Bruch’s membrane and choroidal parenchyma (Figure 1). Malfunction of choroidal macrophages may lead to the development of basal laminar deposits and drusen. Drusen consists of cholesterol-rich lipids and various oxidized proteins, which can induce inflammasome activation (11), and complement activation (12) leading to the death of RPE and photoreceptors, cardinal features of dry AMD.
In wet AMD, the development of macular blood vessels such as choroidal neovascular membrane (CNV) (Figure 1) is driven by abnormal levels of VEGF, which is why intravitreal injection of VEGF inhibitors is effective in treating the disease. Although multiple types of cells in the macula may produce VEGF in wet AMD, macrophage-driven inflammation is known to play a pivotal role (13). Infiltrating macrophages have been detected in human AMD (14). Myeloid cells from AMD patients express higher levels of phosphorylated STAT3 (15) and produce higher levels of VEGF, IL6 and are more proangiogenic when cultured in vitro (16). Macrophages can directly or indirectly participate in retinal angiogenesis in wet AMD.
Macrophages, as an important type of innate immune cells, are supposed to clean debris and promote tissue repair when macula suffers from oxidative damage. Why macrophages become detrimental in AMD still remains elusive.
Lipid metabolism and AMD
Fatty acids such as cholesterol and triglycerides are important fuel source of the retina. In the outer layers of the retina, lipid-rich photoreceptor outer segments are regenerated daily to recycle the light-sensitive visual pigments (e.g., retinal). Photoreceptors maintain the metabolic demands by taking up a large amount of fatty acids through free tatty acid receptor 1 (Ffar1) and very-low-density lipoprotein receptor (VLDLR) (17). The fatty acids are acquired exogenously from choroidal circulation, although they can also be generated endogenously through the glycolysis pathway i.e., acetyl coenzyme A (acetyl-CoA) (18). Dysregulated lipid metabolism is known to play an important role in the development of AMD although the underlying mechanism needs further investigation.
Impaired glucose and fatty acid uptake in photoreceptors results in a reduction in the levels of the Krebs cycle intermediate α-ketoglutarate. Low levels of α-ketoglutarate stabilize hypoxia-induced factor 1α (HIF1α) and induces retinal angiogenesis including wet AMD (17). Although evidence supporting glucose and fatty acid deficiency in AMD barely exists, compelling evidence suggests that dysregulated cholesterol and lipid homeostasis plays a pivotal role in AMD (18). The polymorphisms of genes related to lipid/lipoprotein metabolism have been associated with AMD (18). Higher circulating levels of high-density lipoprotein-cholesterol (HDL-C) are associated with increased risk for AMD (19). HDL can remove cholesterol from cells and is considered to be protective [compared with low density lipoprotein (LDL)]. It is possible that the structure of cholesterol within the HDL in AMD patients may differ from that in healthy controls. Indeed, a recent study by Lin et al. has shown that plasma levels of oxidized cholesterols, the oxysterols, in particular the 24-hydroxycholesterol can discriminate physiological aging from AMD (20). The study highlights the role of oxidized cholesterols in the development of AMD.
Lipid metabolism dysregulation and macrophage malfunction in AMD
Although the pathogenic roles of macrophage malfunction and cholesterol/lipid metabolism dysregulation in AMD have been recognized for over a decade, little is known on how the two phenomena are linked. Macrophages are specialized in metabolizing lipid and this function is needed for them to eliminate cholesterol that they acquire through the phagocytosis of dying cells. On the other hand, macrophage activation and polarization are regulated by the metabolic pathways. For example, the classically activated M1 macrophages are fuelled by the glycolysis pathway, whereas the alternatively activated M2 macrophages relay on fatty acid oxidative phosphorylation (21). The study by Lin and colleagues (20) suggests that macrophage malfunction in AMD may be related to cholesterol metabolism dysregulation. First, the authors showed that mitochondrial oxidative phosphorylation is considerably impaired in aged macrophages. Using transcriptomic approaches, the authors discovered global impairments in cholesterol and lipid homeostasis, including cholesterol/lipid biosynthesis, elimination, transport and uptake (20). Importantly, they found that the intracellular levels of oxidized cholesterol including 4β-hydroxycholesterol, 7-ketocholesterol and cholestane-3β, 5α, 6β-triol were higher in aged macrophages, particularly after treatment with oxidized LDL (20).
This observation is important as accumulation of intracellular cholesterol can lead to activation of several transcription factors, including the liver X receptor α and β (LXRα and LXRβ), the retinoid X receptor (RXR) and member of the peroxisome proliferator-activated receptor (PPAR) family including PPARα and PPARγ (22). Normally, the LXR and RXR would form heterodimers that can upregulate the expression of the ATP-binding cassette subfamily A member 1 (ABCA1) and ABCG1 (22), which can then regulate the efflux of free cholesterol in order to maintain cellular cholesterol homeostasis. This regulatory mechanism appears to be impaired in aged macrophages, particularly in AMD. The expression of ABCA1 and cholesterol efflux is known to be reduced in aged macrophages (23), and ABCA1 polymorphisms are associated with advanced AMD (24). Statins, which inhibits the 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase and reduces cholesterol production from acetyl-CoA, can reduce some high-risk features of AMD (25). Furthermore, activation of the PPARs is also shown to be involved in the development of AMD (26). Taken together, these results suggest that accumulation of intracellular oxysterols in macrophages may confer their pathogenic phenotype leading to the development of AMD.
In the context of AMD, impaired macrophage scavenger function may be related to the development of drusen and pseudo-drusen, whereas uncontrolled macrophage activation towards pro-inflammatory or pro-angiogenic phenotype may lead to the development of dry or wet AMD (Figure 1). Further studies are required to understand why and how cholesterol metabolism is dysregulated and which signalling pathways and functions of macrophage are affected by intracellular oxysterols in AMD.
Acknowledgements
Funding: Research in Dr. M Chen’s laboratory is supported by Fight for Sight (1574/1575) and National Eye Research Centre (SCIAD076). Dr. H Xu’s laboratory is supported by Diabetes UK (11/0004230, 13/0004729), European Union’s Horizon 2020 (722717), Fight for Sight (1361/62; 1425/26; 5057/58), and Dunhill Medical Trust (R188/0211).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lim LS, Mitchell P, Seddon JM, et al. Age-related macular degeneration. Lancet 2012;379:1728-38. [Crossref] [PubMed]
- Coleman HR, Chan CC, Ferris FL 3rd, et al. Age-related macular degeneration. Lancet 2008;372:1835-45. [Crossref] [PubMed]
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106-16. [Crossref] [PubMed]
- Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 2013;120:2292-9. [Crossref] [PubMed]
- Chan CC, Ardeljan D. Molecular pathology of macrophages and interleukin-17 in age-related macular degeneration. Adv Exp Med Biol 2014;801:193-8. [Crossref] [PubMed]
- Guillonneau X, Eandi CM, Paques M, et al. On phagocytes and macular degeneration. Prog Retin Eye Res 2017;61:98-128. [Crossref] [PubMed]
- Xu H, Chen M, Manivannan A, et al. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell 2008;7:58-68. [Crossref] [PubMed]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res 2009;28:348-68. [Crossref] [PubMed]
- Chen M, Xu H. Parainflammation, chronic inflammation, and age-related macular degeneration. J Leukoc Biol 2015;98:713-25. [Crossref] [PubMed]
- Li M, Dolz-Marco R, Messinger JD, et al. Clinicopathologic Correlation of Anti-Vascular Endothelial Growth Factor-Treated Type 3 Neovascularization in Age-Related Macular Degeneration. Ophthalmology 2018;125:276-87. [Crossref] [PubMed]
- Doyle SL, Campbell M, Ozaki E, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med 2012;18:791-8. [Crossref] [PubMed]
- Johnson LV, Forest DL, Banna CD, et al. Cell culture model that mimics drusen formation and triggers complement activation associated with age-related macular degeneration. Proc Natl Acad Sci U S A 2011;108:18277-82. [Crossref] [PubMed]
- Ferguson TA, Apte RS. Angiogenesis in eye disease: immunity gained or immunity lost?. Semin Immunopathol 2008;30:111-9. [Crossref] [PubMed]
- Patel M, Chan CC. Immunopathological aspects of age-related macular degeneration. Semin Immunopathol 2008;30:97-110. [Crossref] [PubMed]
- Chen M, Lechner J, Zhao J, et al. STAT3 Activation in Circulating Monocytes Contributes to Neovascular Age-Related Macular Degeneration. Curr Mol Med 2016;16:412-23. [Crossref] [PubMed]
- Hagbi-Levi S, Grunin M, Jaouni T, et al. Proangiogenic characteristics of activated macrophages from patients with age-related macular degeneration. Neurobiol Aging 2017;51:71-82. [Crossref] [PubMed]
- Joyal JS, Sun Y, Gantner ML, et al. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat Med 2016;22:439-45. [Crossref] [PubMed]
- van Leeuwen EM, Emri E, Merle BMJ, et al. A new perspective on lipid research in age-related macular degeneration. Prog Retin Eye Res 2018;67:56-86. [Crossref] [PubMed]
- Burgess S, Davey Smith G. Mendelian Randomization Implicates High-Density Lipoprotein Cholesterol-Associated Mechanisms in Etiology of Age-Related Macular Degeneration. Ophthalmology 2017;124:1165-74. [Crossref] [PubMed]
- Lin JB, Sene A, Santeford A, et al. Oxysterol Signatures Distinguish Age-Related Macular Degeneration from Physiologic Aging. EBioMedicine 2018;32:9-20. [Crossref] [PubMed]
- Kelly B, O'Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 2015;25:771-84. [Crossref] [PubMed]
- Remmerie A, Scott CL. Macrophages and lipid metabolism. Cell Immunol 2018;330:27-42. [Crossref] [PubMed]
- Sene A, Khan AA, Cox D, et al. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab 2013;17:549-61. [Crossref] [PubMed]
- Neale BM, Fagerness J, Reynolds R, et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc Natl Acad Sci U S A 2010;107:7395-400. [Crossref] [PubMed]
- Vavvas DG, Daniels AB, Kapsala ZG, et al. Regression of Some High-risk Features of Age-related Macular Degeneration (AMD) in Patients Receiving Intensive Statin Treatment. EBioMedicine 2016;5:198-203. [Crossref] [PubMed]
- Herzlich AA, Ding X, Shen D, et al. Peroxisome Proliferator-Activated Receptor Expression in Murine Models and Humans with Age-related Macular Degeneration. Open Biol J 2009;2:141-8. [Crossref] [PubMed]


