Low prevalence of rheumatoid arthritis among patients with pre-existing type 2 diabetes mellitus
Introduction
Rheumatoid arthritis (RA) is the most common autoimmune inflammatory arthritis in adults affecting up to 1% of the population (1). Some of the strongest risk factors for the development of RA are the presence of the human leukocyte antigen (HLA) alleles that contain the “shared epitope”, female gender, family history of RA and tobacco smoke (2,3). In contrast, oral contraceptive use and some dietary constituents might protect from the development of RA (2,3). Interestingly, the impact of diabetes mellitus (DM) on RA incidence is not well established.
DM is a metabolic disorder that is characterized by hyperglycemia. The prevalence of DM has been reported 7% in Greece (4,5) and about 9% globally (6). Several lines of evidence suggest attenuation of immune response in patients with DM (7-9). For example, DM per se confers an increased rate and severity of infections to these patients comparing to people without DM (7). Advanced glycation end-products (AGEs), which are formed because of hyperglycemia, may alter the expression and secretion of interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α and inflammatory mediators, through a specific interaction with membrane receptors expressed on T-lymphocytes, monocytes and macrophages (8,9). In addition, glycation of immunoglobulins and/or their receptors in patients with chronic hyperglycemia may downregulate the immune system’s capacity to respond to antigenic stimuli (10).
Based on the above we examined previously the hypothesis that pre-existing hyperglycemia may have a protective effect on the development of RA (11), a disease in which the earliest event is an intense inflammatory response to unknown stimuli within the joint. In a preliminary study we found that in patients with type 1 DM (T1DM), which coexists with other autoimmune diseases including RA (12), the prevalence of RA was higher in patients with T1DM compared with age- and gender-matched controls without DM (0.58% vs. 0.39%, respectively) (11). In contrast, RA prevalence was lower in patients with type 2 DM (T2DM) (0.25% vs. 0.83% in controls), albeit the difference did not reach statistical significance (11). Apart from our study, there are a few previous studies that have examined the relationship between DM and RA with conflicting results (13-15).
The aim of this study was to examine the prevalence of RA among a large cohort of well characterized and followed up patients with T2DM.
Methods
Participants
A total of 3,260 participants were recruited for the study; half of them (n=1,630) had pre-existing T2DM and were recruited consecutively from the two outpatient diabetes centers based at the National and Kapodistrian University of Athens Medical School and were examined for the presence of RA. All patients were regularly followed-up for at least 10 years post-diagnosis. Diagnosis and classification of DM and RA was based on the American Diabetes Association criteria (16) and the American College of Rheumatology criteria (17), respectively. The study was approved by the ethics committee of our Institution (approval ID No. 1452/2015) and conducted according to the principles of the Declaration of Helsinki (18).
The rest 1,630 non-diabetic subjects were randomly selected from the participants in the ESORDIG study, a large population-based door-to door study conducted by qualified rheumatologists who examined the prevalence of rheumatic diseases in our country (19). Control subjects without DM were matched 1:1 for age and gender with patients with T2DM and were studied in parallel. To avoid misclassification of subjects with steroid-induced DM as T2DM, we excluded from the study patients with RA and controls who had taken corticosteroids for longer than one month for any reason in the year prior to recording for the present study.
Statistical analysis
The ESORDIG study revealed that the prevalence of RA in the general population of Greece is 0.68% (19). Based on our preliminary data (11) and assuming a difference in proportions in the prevalence of RA of 0.53 between subjects without and with T2DM, a sample size of 1,000 subjects per group could provide a power of 95% at a two-tailed α=0.01 and 99% CI 0.53–0.63 to yield a statistically significant difference in the prevalence of RA among participants with and without T2DM.
The Statistical Package for the Social Sciences (IBM SPSS software version 22.0 for Windows, Armonk, NY, USA) was used for analyses. The Power and Precision Software (Biostat, version 2.0, Englewood, NJ, USA) was used for the calculation of the power of the study. All variables were tested for normal distribution of the data using the Kolmogorov-Smirnov test. Differences between parametric variables were examined using the student-t-test. The chi-square test was used to assess differences in categorical variables. P values <0.05 (two-tailed) were considered statistically significant.
Results
The demographic and clinical characteristics of the study participants are classified according to the presence of DM in Table 1. The gender, the age and the age subgroups of the study participants were fully matched. More specifically, in both groups 909 subjects were male (55.8%) and 721 (44.2%) were female. The mean age of the males was 66.5±11.9 and 66.2±11.4 years for the diabetes- and the non-diabetes group, respectively, while the mean age of the females was 66.4±12.1 and 66.1±11.8 years for the diabetes- and the non-diabetes group, respectively. The age groups of the participants are shown in Table 1. The median duration among people with DM was 16 years, the mean body mass index (BMI) for males with DM was 27.5±4.1 kg/m2, while the mean BMI for males without DM was 26.4±3.4 kg/m2. Regarding women, the mean BMI was 29.6±5.9 kg/m2 for subjects with DM and 26.3±4.3 kg/m2, for participants without DM.
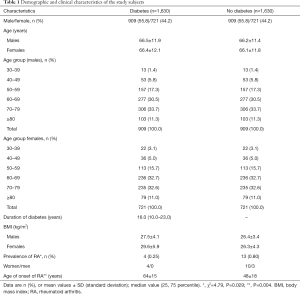
Full table
The prevalence of RA in the group without DM was 0.80% (13 subjects; 10 women, 3 men), while the prevalence of RA in the diabetes group was 0.25% (4 subjects, all women) (χ2=4.79, P=0.029). The OR (95% CI) for subjects with T2DM to have RA in comparison with the subjects without DM was 0.30 (0.10–0.64), P=0.038.
Three out of the 4 cases of RA were diagnosed in the first 5 years while 1 case of RA was diagnosed 8 years after DM onset. The mean (± SD) age of RA diagnosis was 48±18 years in the non-diabetes and 64±15 years in the DM group (P=0.004, Table 1).
Discussion
In the present study, we demonstrated that the prevalence of RA is lower and occurs in an older age in patients with pre-existing T2DM in comparison with people without T2DM.
RA is a chronic inflammatory disease in which the earliest event is an intense inflammatory response to unknown stimuli within the joint (3,20). Inflammatory mediators such as CRP, IL-6, and TNF-α are frequently elevated in the serum of patients many years before the clinical onset of RA and correlate with the disease activity, suggesting a critical role in the immunopathogenesis and progression of RA (21). IgG is the most abundant immunoglobulin isotype comprising ~75% of the total serum immunoglobulins (20). IgG triggers its effector function, that is complement activation, via its Fc (22). Previous studies reported that there is abundant codeposition of IgG containing immunocomplexes due to antibody-cartilage surface interaction and activated complement components in the joints of more than 90% of RA patients, suggesting that immunoglobulins and complement activation are strongly involved in the pathogenesis of RA (23-25).
The question is whether alterations in the activity of immune system as a result of hyperglycemia in patients with pre-existing T2DM can modulate the inflammatory process and alter the risk of development of RA.
It is known that DM undermines host defenses increasing the susceptibility to viral, bacterial and fungal infections mainly through modulation of the immune system (26). Thus, hyperglycemia attenuates inflammation induced neutrophil activation (27). In addition, hyperglycemia reduces neutrophil degranulation, chemotaxis, phagocytosis and bactericidal capacity (27,28). These effects are due mainly to changes in neutrophil metabolism through the inhibition of glucose-6-phosphate dehydrogenase (G6PD) and activation of protein kinase C (26). In addition, hyperglycemia can inhibit complement receptor and Fc-γ receptor-mediated phagocytosis by normal human neutrophils by activating PKC-α or PKC-β or both (29). Hyperglycemia-induced protein kinase C activation inhibits phagocytosis of C3b- and immunoglobulin g-opsonized yeast particles in normal human neutrophils (29). Moreover, hyperglycemia inhibited complement receptor and Fc-γ receptor-mediated phagocytosis of opsonized yeast particles by normal human neutrophils in vitro (30). Acute hyperglycemia inhibits opsonization when glucose binds directly to the biochemically active site of the third component of complement C3 in a nonenzymatic reaction and blocks its attachment to the microbial surfaces (31). Hyperglycemia can also cause nonenzymatic glycosylation of immunoglobulins; this reduces complement fixation and opsonization of microbes (26). Furthermore, in vitro studies have shown that even transient hyperglycemia causes a significant reduction in complement fixation by immunoglobulin (30). The results of the aforementioned studies indicate that patients with DM have reduced immunological response to viral, fungal and bacterial infections through the effects of hyperglycemia on complement receptor and Fc receptor-mediated phagocytosis. Although the pathogenesis of RA is unknown, previous reports have shown that the initiating event can be an intense immune response to noxious stimuli including infectious agents (32). It has been proposed that microbial products in the synovial fluid may generate a chronic inflammatory response; alternatively, the microorganism or the response to the microorganism might induce an immune response to components of the joint or it might prime the host to cross-reactive determinants expressed within the joint as a result of “molecular mimicry” (32). A reduced immune response in the context of DM to infectious agents could explain the protective effect of DM on the pathogenesis of the disease.
Previous studies have shown that all immunoglobulins are glycated both in vitro and in vivo under exposure to high glucose concentrations in both the Fc and the Fab fragments (33). It is known that changes due to modification in the Fc region of immunoglobulins such as glycosylation influences the effector function of IgG mediated by its receptor and activates the classical pathway of the complement system (34-38). Glycosylation of the Fc is characterized by presence of a single chain N-linked glycan attached to each heavy chain at asparagine. It has been demonstrated that the lack of glycation on the Fc-N-linked glycans increases the inflammatory capacity of IgG in mice while glycation attenuates this activity (39-42). IgG in RA patients contains less glycans such as galactose and sialic acid (43). Noteworthy, the glycosylation pattern of anti-citrullinated protein antibodies (ACPA) changes before the onset of RA skewing toward more inflammation (44,45). Moreover, pregnancy-induced spontaneous improvement of RA has been linked to pregnancy-related reduction in the glycosylation of IgGs (46). These data support the concept that patients with DM may be protected from the development of RA because posttranslational glycation of immunoglobulins, as a result of hyperglycemia, make them less immunogenic.
In addition, the adipose tissue-derived factor leptin has been emerged as a key regulator of nutritional state and metabolism, as well as a modulator of immune system activation and innate-adaptive frontier (47). Noteworthy, experimental findings have shown that antigen-induced arthritis is markedly attenuated in either ob/ob or db/db mice models of T2DM that lack leptin or leptin receptors, respectively (47). This finding further supports the potential protective effect of T2DM on development of RA.
There are a few previous studies that examined the association between RA and DM. Simard et al. in the United States, found that the adjusted OR for the cross-sectional association between RA and diabetes was ranging from 1.1 to 1.5, which was not significant (14); in this study there were included noninstitutionalized civilians older than 60 years old. These findings do not contradict the findings presented herein, because DM is expected to occur in more than 15% in an American population at this age, whereas subjects with RA and either steroid-induced or concurrent autoimmune T1DM were not excluded (14). Moreover, Lu et al. demonstrated that among subjects with T2DM the risk of developing RA was significantly higher in female (OR: 1.46, 95% CI: 1.24–1.72), but not in male participants (13). However, in this study, most of the population were females (77.4%), while in our study only 44.2% were female; it is known that RA is more common in women (2). Additionally, the population of this study was ten years younger than the population of our study.
Furthermore, Liao et al. reported that the prevalence of pre-existing T2DM among 1,419 incident RA patients in Sweden was 2.96% vs. 2.75% in 1,674 non-RA controls matched by age, sex and location of residence (15). A reason that partly explains the discrepancy between their results and those of our study is the possibility that information on DM status was not available in 27 of 113 subjects with self-reported DM. DM status was acquired by telephone contact, whereas steroid-induced DM was probably not excluded. Moreover, the RA population studied by Liao et al. was much younger than our diabetic population (92% vs. 56% of subjects, respectively, being between 30 and 70 years old) and was predominantly female (71% vs. 44%, respectively) (15). On the other hand, the established specific association between T1DM and APCA positive RA in this study (15) is compatible with the higher than expected prevalence of RA among patients with T1DM in our country (11). We excluded individuals with T1DM from the present study because previous reports have shown that T1DM and RA have some common underlying genetic elements (HLA A1, DR3, and DR4) that may predispose to more frequent coexistence of these diseases (15).
Strength of this study is that we used robust data from the 10-year prospective follow-up of well-characterized participants. We found that the prevalence of RA was significantly lower in patients with T2DM and it occurs early after DM diagnosis. This finding further supports the hypothesis that immunosuppression in patients with long-standing DM may be involved in the lower prevalence of RA among subjects with T2DM.
The main limitation of this study is the cross-sectional design that provides associations between DM and RA, but does not explain the underlying pathophysiology neither implies a cause and effect relationship between these two diseases. A prospective study is required to examine the impact of T2DM on RA development and evolution. Another limitation may be the relatively small number of participants included, which makes the findings of the study to be statistically significant but not clinically meaningful, given the low prevalence of RA. However, we tried to avoid type I or type II statistical errors by using proper statistical testing before the initiation of the study.
To sum up, a significantly lower than expected prevalence of RA was found in patients with (non-autoimmune) T2DM. A decreased risk of first-ever diagnosis of RA in association with the presence of DM in the previous year was also reported recently by Rodríguez et al., who examined the incidence of RA in the United Kingdom and investigated associations with consultation behavior, risk factors, and comorbidities (48). Alterations in immune responses due to glycation, or AGEs-mediated interactions, and/or other factors that affect the complex (auto)immune mechanisms underlying RA development may account for the negative link described herein.
RA is a complex disease and its pathogenesis has not been elucidated so far; however, it is established that the disease has a strong immunopathogenic background. On the other hand, T2DM is common, and its prevalence and incidence are increasing globally. Hyperglycemia affects in many ways the immune system. Clearly, more clinical and basic research is necessary to elucidate the relationship between RA and DM and the mechanisms mediating the effects of DM on the development and progression of RA as well as other chronic inflammatory diseases.
Conclusions
Prevalence of RA is significantly lower in subjects with T2DM. This finding implies that hyperglycemia through immunosuppression might have a protective role for the development of RA.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committees of the Laiko General Hospital and the Hippokration General Hospital (approval ID No. 1452/2015) and written informed consent was obtained from all individuals before participation in the study.
References
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 2008;58:15-25. [Crossref] [PubMed]
- Deane KD. Can rheumatoid arthritis be prevented? Best Pract Res Clin Rheumatol 2013;27:467-85. [Crossref] [PubMed]
- Calabresi E, Petrelli F, Bonifacio AF, et al. One year in review 2018: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 2018;36:175-84. [PubMed]
- Liatis S, Dafoulas GE, Kani C, et al. The prevalence and treatment patterns of diabetes in the Greek population based on real-world data from the nation-wide prescription database. Diabetes Res Clin Pract 2016;118:162-7. [Crossref] [PubMed]
- Tentolouris A, Eleftheriadou I, Athanasakis K, et al. Prevalence of diabetes mellitus as well as cardiac and other main comorbidities in a representative sample of the adult Greek population in comparison with the general population. Hellenic J Cardiol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed. Brussels, Belgium: International Diabetes Federation, 2017.
- Gupta S, Koirala J, Khardori R, et al. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am 2007;21:617-38. [Crossref] [PubMed]
- Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 1988;318:1315-21. [Crossref] [PubMed]
- Thornalley PJ. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol (Noisy-le-grand) 1998;44:1013-23. [PubMed]
- Jaleel A, Halvatsiotis P, Williamson B, et al. Identification of Amadori-modified plasma proteins in type 2 diabetes and the effect of short-term intensive insulin treatment. Diabetes Care 2005;28:645-52. [Crossref] [PubMed]
- Tentolouris N, Arapostathi C, Voulgari C, et al. The effect of diabetes mellitus on the prevalence of rheumatoid arthritis: a case-control study. Diabet Med 2008;25:1010-1. [Crossref] [PubMed]
- Somers EC, Thomas SL, Smeeth L, et al. Are individuals with an autoimmune disease at higher risk of a second autoimmune disorder? Am J Epidemiol 2009;169:749-55. [Crossref] [PubMed]
- Lu MC, Yan ST, Yin WY, et al. Risk of rheumatoid arthritis in patients with type 2 diabetes: a nationwide population-based case-control study. PLoS One 2014;9. [Crossref] [PubMed]
- Simard JF, Mittleman MA. Prevalent rheumatoid arthritis and diabetes among NHANES III participants aged 60 and older. J Rheumatol 2007;34:469-73. [PubMed]
- Liao KP, Gunnarsson M, Kallberg H, et al. Specific association of type 1 diabetes mellitus with anti-cyclic citrullinated peptide-positive rheumatoid arthritis. Arthritis Rheum 2009;60:653-60. [Crossref] [PubMed]
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2015;38 Suppl:S8-16. [Crossref] [PubMed]
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315-24. [Crossref] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Andrianakos A, Trontzas P, Christoyannis F, et al. Prevalence and management of rheumatoid arthritis in the general population of Greece--the ESORDIG study. Rheumatology (Oxford) 2006;45:1549-54. [Crossref] [PubMed]
- Holers VM, Banda NK. Complement in the Initiation and Evolution of Rheumatoid Arthritis. Front Immunol 2018;9:1057. [Crossref] [PubMed]
- van Steenbergen HW, Huizinga TW, van der Helm-van Mil AH. The preclinical phase of rheumatoid arthritis: what is acknowledged and what needs to be assessed? Arthritis Rheum 2013;65:2219-32. [Crossref] [PubMed]
- Koro C, Bielecka E, Dahl-Knudsen A, et al. Carbamylation of immunoglobulin abrogates activation of the classical complement pathway. Eur J Immunol 2014;44:3403-12. [Crossref] [PubMed]
- Cooke TD, Hurd ER, Jasin HE, et al. Identification of immunoglobulins and complement in rheumatoid articular collagenous tissues. Arthritis Rheum 1975;18:541-51. [Crossref] [PubMed]
- Nakagawa K, Sakiyama H, Tsuchida T, et al. Complement C1s activation in degenerating articular cartilage of rheumatoid arthritis patients: immunohistochemical studies with an active form specific antibody. Ann Rheum Dis 1999;58:175-81. [Crossref] [PubMed]
- Holers VM. Complement and its receptors: new insights into human disease. Annu Rev Immunol 2014;32:433-59. [Crossref] [PubMed]
- Jafar N, Edriss H, Nugent K. The Effect of Short-Term Hyperglycemia on the Innate Immune System. Am J Med Sci 2016;351:201-11. [Crossref] [PubMed]
- Stegenga ME, van der Crabben SN, Blumer RM, et al. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood 2008;112:82-9. [Crossref] [PubMed]
- Wierusz-Wysocka B, Wysocki H, Wykretowicz A, et al. The influence of increasing glucose concentrations on selected functions of polymorphonuclear neutrophils. Acta Diabetol Lat 1988;25:283-8. [Crossref] [PubMed]
- Saiepour D, Sehlin J, Oldenborg PA. Hyperglycemia-induced protein kinase C activation inhibits phagocytosis of C3b- and immunoglobulin g-opsonized yeast particles in normal human neutrophils. Exp Diabesity Res 2003;4:125-32. [Crossref] [PubMed]
- Hennessey PJ, Black CT, Andrassy RJ. Nonenzymatic glycosylation of immunoglobulin G impairs complement fixation. JPEN J Parenter Enteral Nutr 1991;15:60-4. [Crossref] [PubMed]
- Mauriello CT, Hair PS, Rohn RD, et al. Hyperglycemia inhibits complement-mediated immunological control of S. aureus in a rat model of peritonitis. J Diabetes Res 2014;2014. [Crossref] [PubMed]
- Picerno V, Ferro F, Adinolfi A, et al. One year in review: the pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 2015;33:551-8. [PubMed]
- Wei B, Berning K, Quan C, et al. Glycation of antibodies: Modification, methods and potential effects on biological functions. MAbs 2017;9:586-94. [Crossref] [PubMed]
- Brady LJ, Velayudhan J, Visone DB, et al. The criticality of high-resolution N-linked carbohydrate assays and detailed characterization of antibody effector function in the context of biosimilar development. MAbs 2015;7:562-70. [Crossref] [PubMed]
- Malhotra R, Wormald MR, Rudd PM, et al. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med 1995;1:237-43. [Crossref] [PubMed]
- Nose M, Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A 1983;80:6632-6. [Crossref] [PubMed]
- Thomann M, Schlothauer T, Dashivets T, et al. In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity. PLoS One 2015;10. [Crossref] [PubMed]
- Vestrheim AC, Moen A, Egge-Jacobsen W, et al. Different glycosylation pattern of human IgG1 and IgG3 antibodies isolated from transiently as well as permanently transfected cell lines. Scand J Immunol 2013;77:419-28. [Crossref] [PubMed]
- Anthony RM, Nimmerjahn F. The role of differential IgG glycosylation in the interaction of antibodies with FcgammaRs in vivo. Curr Opin Organ Transplant 2011;16:7-14. [Crossref] [PubMed]
- Banda NK, Wood AK, Takahashi K, et al. Initiation of the alternative pathway of murine complement by immune complexes is dependent on N-glycans in IgG antibodies. Arthritis Rheum 2008;58:3081-9. [Crossref] [PubMed]
- Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006;313:670-3. [Crossref] [PubMed]
- Karsten CM, Pandey MK, Figge J, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med 2012;18:1401-6. [Crossref] [PubMed]
- Parekh RB, Dwek RA, Sutton BJ, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 1985;316:452-7. [Crossref] [PubMed]
- Ercan A, Cui J, Chatterton DE, et al. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum 2010;62:2239-48. [Crossref] [PubMed]
- Rombouts Y, Ewing E, van de Stadt LA, et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis 2015;74:234-41. [Crossref] [PubMed]
- van de Geijn FE, Wuhrer M, Selman MH, et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther 2009;11:R193. [Crossref] [PubMed]
- Busso N, So A, Chobaz-Peclat V, et al. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol 2002;168:875-82. [Crossref] [PubMed]
- Rodríguez LA, Tolosa LB, Ruigómez A, et al. Rheumatoid arthritis in UK primary care: incidence and prior morbidity. Scand J Rheumatol 2009;38:173-7. [Crossref] [PubMed]


