
Treating atherosclerosis: targeting risk factors should not be the only option
Cardiovascular mortality increases continuously
Myocardial infarction, stroke and peripheral arterial disease are the leading cause of mortality and multimorbidity worldwide. Numbers are even more dramatic when considering deaths attributed to classical risk factors (e.g., diabetes, hypertension…). However, the clinical approach mostly based on statins and reducing risks, has not changed over time. Certainly, aggressive and efficient targeting of preventable causes of atherosclerotic vascular disease (AVD) may reduce the growing burden of disease but results, partially due to underdiagnosis and/or undertreatment, are disappointing typically in older adults (1). Consequently, global deaths from AVD have increased by 41% between 1990 and 2013 despite a 39% decrease in age-specific death rates (2). It is important to remember that life expectancy is higher than 70 years worldwide and perhaps it is time to consider substantial changes in strategies. For instance, early detection and intervention, even using invasive actions, seems effective and currently implemented in most countries for cancer. Advisability and appraisal of such procedures in atherosclerosis currently remain just a matter of scholar debate. Surprisingly, personalized assessment of subclinical atherosclerosis and the subsequent intervention remain anecdotic when current imaging technologies appear to be adequate to fit the challenge to reduce the preponderance of AVD among health problems (3).
Beyond lifestyle: knowledge is far from complete
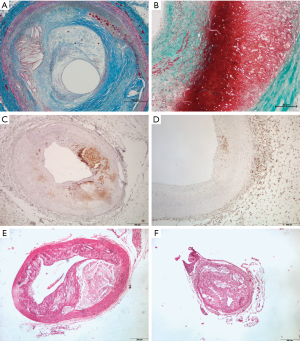
Medical and surgical treatments for advanced AVD manage some of their more immediate consequences but they do little to prevent, halt or reverse atherosclerosis. Tobacco and alcohol cessation, nutritional intervention, increase in physical activity, stress reduction, and maintenance of healthy cholesterol profiling have positive effects as demonstrated in clinical studies. Results in the real world are not so positive; cruelly resembling the situation observed with the obesity epidemics, i.e., causal links between atherosclerosis and the risk of disease are not simple (4). It is knowledge that is the key in approaching complex diseases in which problems are hard but likely to be solved. No informed person can deny that damage to arteries from lifestyle variables are harmful and that they are continuous and we do nothing damage will likely become catastrophic. In atherosclerosis, the original triggering event causes endothelial dysfunction and/or endothelial injury. That event is often distant, probably in childhood and nutrient related, but dyslipidemia and/or hypertension may later cause re-injury making the artery susceptible to further stages in atherogenesis. Treatment of these conditions is mandatory although does not represent a truly healing procedure (5). Aging is also a powerful determinant but mechanisms driving atherosclerosis development with age, beyond prolonged exposure to risk factors, remain unclear. Oxidative stress and inflammation result in long-standing cellular metabolic abnormalities, which rarely reverse. Over time, these unaltered mechanisms reinforce the concept that the disease is actually a whole body disease, which is progressive (6). Inflammatory cells, in an attempt to heal, may gain entry to the vessel wall via circulation or the adventitial vasa vasorum but vascular inflammation enhances lipid deposition in the subendothelial layer of arteries and retention in smooth muscle cells entail structural changes stimulating transformation of macrophages into foam cells in a self-maintaining progressive cycle (7,8). Advanced atherosclerotic lesions are replete of both cholesterol and immune cells (Figure 1A,B,C,D). Atherosclerosis appears to be a matter of unresolved inflammation with very few useful advances since the seminal observations of Rudolph Virchow [1821–1902]. Efforts to modulate the inflammatory response in atherosclerosis have been scarce and challenging mainly because off-target effects of available agents may offset the potentially beneficial effects (9). Therefore, in treating or regressing atherosclerosis to remove cholesterol is essential and in humans reverse cholesterol transport (RCT) pathways may reduce the amount of plaque in vessel walls.
RCT and cholesterol efflux
High-density lipoproteins (HDL), especially hepatic nascent HDL particles, are key factors in RCT, a mechanism by which cholesterol is transported from arteries to the liver for excretion from the body. Briefly, ATP-binding cassette transporter A1 (ABCA1) and similar molecules translocate cholesterol to the cell surface and form lipid domains with the ability to interact with apolipoproteins. HDL-ApoA-I stimulates the enzymatic production of cholesteryl esters that migrate to the core of the HDL particle to become mature HDL. Cholesterol esters are then exchanged for triglycerides in ApoB-containing lipoproteins [low density lipoproteins (LDL)] and taken up by liver cells through both HDL and LDL specific receptors (10). RCT from macrophages to liver and ultimately biliary excretion might explain the beneficial effects of HDL in atherosclerosis. Impairment in genes involved in cholesterol efflux and low serum levels of HDL correlate with both premature atherosclerosis and faster atherosclerosis progression. The nearly obvious consequence was to consider that raising HDL-cholesterol levels could be a potential therapeutic target but clinical trials have failed to decrease mortality; implications have been reviewed and are still being considered (11). The failure of clinical trials does not necessarily preclude the progression of plausible ideas. HDL-cholesterol does not define HDL function and proteins or other lipids in HDL could be the actual mediators. Removal of cholesterol in arteries is a necessary, although probably insufficient, factor for any successful treatment of atherosclerosis and there is consensus on the efficacy of apoA-I in improving (or preventing) atherosclerosis (12). However, to synthesize human apoA-I (243 amino acid residues) as a therapeutic candidate is a difficult and expensive task, which requires repeated intravenous administration for a long period of time. Simply put, unaffordable for treatment of millions of patients. Then, research was directed towards finding methods to modify the lipid and protein cargo of HDL through apolipoprotein mimetic peptides and results suggested the promotion of cholesterol efflux and improvement in anti-inflammatory effects.
HDL mimetics and apolipoprotein A-I mimetic peptides
The seminal idea of designing a simple, short, peptide mimicking the class A amphipathic helixes contained in apoA-I is not new (13). Subtle differences in activity of the obtained peptides apparently depend on the configuration of the hydrophobic face of the peptides, i.e., the number, either four or five, of phenylalanine (F) residues. Chemical details have been reviewed (14) but 4F (Ac-D-W-F-K-A-F-Y-D-K-V-A-E-K-F-K-E-A-F-NH2) appears to be the most studied peptide with the ability to synergize the use of statins in humans (15). These peptides have been tested in a large number of animal models of disease suggesting multifunctional mechanisms (16) and results support active research on strategies to use HDL mimetics as therapeutic agents. For instance, minor variations in apolipoprotein mimetic peptides modulate cholesterol transport, suppress inflammation and improve glucose-insulin tolerance (17). Pharmaceutical industries have been historically reluctant to promote these medications because they usually require intravenous administration and are expensive. Some binding characteristics that allow oral delivery of peptides and the development of multivalent constructs with different peptide and lipid proportions, nanometer-scale discoidal HDL-like particles with desirable pharmacokinetic profiles, have increased attention on such medications. Results suggest potential use upon mimicking the function of mediators involved in pathologic processes during vascular damage and to elucidate plaque composition through imaging techniques (18). Peptides that did not need end-blocking groups for efficacy have additionally reduced cost of production (19).
Synthetic HDL-mediated drug delivery
Recently, major advances have been achieved by using ApoA-I mimetic peptides with the ends uncapped (PVLDLFRELLNELLEALKQKLK) combined with 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine and 1,2-dimyristoyl-sn-glycero-3-phosphocholine (19). These manipulations represent a novel nanoparticle-mediated drug delivery system based on synthetic HDL (sHDL), which was originally developed as an acute infusion treatment to rapidly mobilize cholesterol from the atheroma, reduce plaque volume and decrease the probability of a second cardiac event (20). These concepts and the fact that HDLs are multifunctional nanoparticles could be generalized towards rational design of peptide-based therapeutics. Compared with other synthetic nanocarriers, such as liposomes, micelles, and inorganic and polymeric nanoparticles, sHDLs have beneficial attributes that include their ultrasmall size, high tolerability, long circulating half-life, and intrinsic targeting properties to different recipient cells (21). Taken together, findings suggest a general strategy for personalized nanomedicine. For instance, HDL-mimicking nanodiscs coupled with antigen peptides represent a powerful approach for cancer immunotherapy (22).
In atherosclerosis, increasing cholesterol acceptors is a failed approach probably because levels of ABCA1 in advanced atherosclerotic plaques are markedly reduced and this likely deleterious effect is further enhanced by statins (23). Contrarily, increasing RCT is a well-documented strategy. As expected, sHDL nanoparticles encapsulating LXR agonists to upregulate the expression of ABC transporters, RCT and cholesterol efflux were increased in macrophages using in vitro models (19,24). In vivo, using animal models, sHDLs successfully delivered the LXR agonist to the atheroma and activated the expression of LXR target genes in atherosclerotic plaques without side effects on hepatic lipogenesis. Finally, they also observed either a trend towards atherosclerosis regression or significant regression upon different measurements of atherosclerosis. In this particular point, the limitations of mice as atherosclerotic models are important, as illustrated in Figure 1E,F and previously indicated (25). Findings strongly suggest that development of a multifunctional nanocarrier targeting at plaque macrophages to enhance cholesterol efflux represents an efficient strategy for the treatment and prevention of atherosclerosis. Therefore, this strategy warrants and deserves further investigation translationally.
Conclusions
There is a high socioeconomic, medical and scientific interest to find strategies for intervention and/or treatment on atherosclerosis. Amelioration of contributing risk factors and underlying processes is not sufficient in a world in which healthspan is lower than life expectancy. New approaches to treat cardiovascular diseases are urgently required and apolipoprotein mimetics may have a promising therapeutic potential. Searching practical methods of producing and administering these agents is paramount and improvements in technology and synthesis platforms may reverse the reluctance of pharmaceutical industry. Recent investigations on sHDL nanoparticles encapsulating LXR agonists may help to select the best targets to take forward on atherosclerosis regression. Pursuing this goal, synthetic HDLs act not only as a cholesterol acceptor but also as a LXR agonist delivery vehicle that specifically target ABC transporters in macrophages improving RCT. We envision this is a perfect example on how research focused on causality might indicate the possibility of intervention and define an appropriate prevention policy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Forman DE, Maurer MS, Boyd C, et al. Multimorbidity in Older Adults With Cardiovascular Disease. J Am Coll Cardiol 2018;71:2149-61. [Crossref] [PubMed]
- Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 2015;372:1333-41. [Crossref] [PubMed]
- Fernández-Friera L, Ibáñez B, Fuster V. Imaging subclinical atherosclerosis: is it ready for prime time? A review. J Cardiovasc Transl Res 2014;7:623-34. [Crossref] [PubMed]
- Parsons C, Agasthi P, Mookadam F, et al. Reversal of coronary atherosclerosis: Role of life style and medical management. Trends Cardiovasc Med 2018;28:524-31. [Crossref] [PubMed]
- Hurtubise J, McLellan K, Durr K, et al. The Different Facets of Dyslipidemia and Hypertension in Atherosclerosis. Curr Atheroscler Rep 2016;18:82. [Crossref] [PubMed]
- Hernández-Aguilera A, Nielsen SH, Bonache C, et al. Assessment of extracellular matrix-related biomarkers in patients with lower extremity artery disease. J Vasc Surg 2018;68:1135-42.e6. [Crossref] [PubMed]
- Viola J, Soehnlein O. Atherosclerosis - A matter of unresolved inflammation. Semin Immunol 2015;27:184-93. [Crossref] [PubMed]
- Dubland JA, Francis GA. So Much Cholesterol: the unrecognized importance of smooth muscle cells in atherosclerotic foam cell formation. Curr Opin Lipidol 2016;27:155-61. [Crossref] [PubMed]
- Raggi P, Genest J, Giles JT, et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018;276:98-108. [Crossref] [PubMed]
- Ohashi R, Mu H, Wang X, et al. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM 2005;98:845-56. [Crossref] [PubMed]
- Lüscher TF, Landmesser U, von Eckardstein A, et al. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ Res 2014;114:171-82. [Crossref] [PubMed]
- Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 2003;290:2292-300. [Crossref] [PubMed]
- Anantharamaiah GM, Jones JL, Brouillette CG, et al. Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J Biol Chem 1985;260:10248-55. [PubMed]
- Navab M, Anantharamaiah GM, Reddy ST, et al. Apolipoprotein A-I mimetic peptides. Arterioscler Thromb Vasc Biol 2005;25:1325-31. [Crossref] [PubMed]
- Bloedon LT, Dunbar R, Duffy D, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res 2008;49:1344-52. [Crossref] [PubMed]
- Reddy ST, Navab M, Anantharamaiah GM, et al. Searching for a successful HDL-based treatment strategy. Biochim Biophys Acta 2014;1841:162-7. [Crossref] [PubMed]
- Recio C, Maione F, Iqbal AJ, et al. The Potential Therapeutic Application of Peptides and Peptidomimetics in Cardiovascular Disease. Front Pharmacol 2017;7:526. [Crossref] [PubMed]
- Zhao Y, Imura T, Leman LJ, et al. Mimicry of high-density lipoprotein: functional peptide-lipid nanoparticles based on multivalent peptide constructs. J Am Chem Soc 2013;135:13414-24. [Crossref] [PubMed]
- Guo Y, Yuan W, Yu B, et al. Synthetic High-Density Lipoprotein-Mediated Targeted Delivery of Liver X Receptors Agonist Promotes Atherosclerosis Regression. EBioMedicine 2018;28:225-33. [Crossref] [PubMed]
- Di Bartolo BA, Nicholls SJ, Bao S, et al. The apolipoprotein A-I mimetic peptide ETC-642 exhibits anti-inflammatory properties that are comparable to high density lipoproteins. Atherosclerosis 2011;217:395-400. [Crossref] [PubMed]
- Kuai R, Li D, Chen YE, et al. High-Density Lipoproteins: Nature's Multifunctional Nanoparticles. ACS Nano 2016;10:3015-41. [Crossref] [PubMed]
- Kuai R, Ochyl LJ, Bahjat KS, et al. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater 2017;16:489-96. [Crossref] [PubMed]
- Wong J, Quinn CM, Gelissen IC, et al. The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis 2008;196:180-9. [Crossref] [PubMed]
- Im SS, Osborne TF. Liver x receptors in atherosclerosis and inflammation. Circ Res 2011;108:996-1001. [Crossref] [PubMed]
- Joven J, Rull A, Ferré N, et al. The results in rodent models of atherosclerosis are not interchangeable: the influence of diet and strain. Atherosclerosis 2007;195:e85-92. [Crossref] [PubMed]


