Sole causal therapy worsens outcome as compared to no therapy and combined causal and goal-directed supportive therapy in ovine septic shock
Introduction
Sepsis and septic shock are among the most common causes of death in intensive care units (1,2). To investigate and establish new therapeutic agents and strategies, preclinical sepsis models are necessary that implement current treatment guidelines thereby allowing the most reliable translational research (3).
Though many components of sepsis treatment have changed over the years, causal therapy and supportive hemodynamic treatment remain the basis of sepsis treatment (4,5). Prospective randomized investigations concerning omission or delay of established components of sepsis treatment are rare for obvious ethical reasons. However, due to analyses of Kumar et al. (6) and other investigators (7,8) we know that a delay in appropriate antibiotic treatment may result in increased mortality and this knowledge is accepted as “state of the art”. A retrospective study of Seymour et al. analyzed the median time of initiation of broad-spectrum antibiotics and completion of intravenous fluid bolus regarding mortality (9). Interestingly, the authors recognized impaired survival in patients who received delayed antibiotic therapy while delayed completion of intravenous fluid bolus had no influence on survival. The current evidence for fluid resuscitation in sepsis remains highly conflicting (10) and findings like the results from the trials of Seymour and Andrews indicate that even established concepts in critical care medicine should be re-evaluated. Therefore, the present study aimed to evaluate the effects of causal sepsis therapy with absence of hemodynamic support versus combined causal and supportive therapy as well as the natural evolution of sepsis on hemodynamics, organ function variables as well as survival in septic shock. For this purpose, a clinically relevant large animal model of ovine septic shock including causal and supportive hemodynamic therapy as suggested by the current sepsis guidelines was established (4,5).
We hypothesized that combined causal and supportive hemodynamic therapy improve survival and organ function as compared to natural evolution of sepsis or causal treatment only in a large animal model of septic shock.
Methods
Study approval
The present study was approved by the Animal Care Committee of the State Government of North-Rhine Westphalia (LANUV NRW, Recklinghausen, Germany) with the approval no. 84-02.04.2011.A300. In addition, the responsible veterinarians of the facility were consulted before the beginning of the study about the adequate dosage of all used medications. All methods were performed in accordance with the National Institutes of Health Guide and as well as the American Physiologic Society’s “Guide for the Care and Use of Laboratory Animals” using established protocols.
Anesthesia
After withdrawal of food for 12 hours, twenty healthy, female ewes aged 6–9 months [41.0 kg (35.0–43.0)] were anesthetized by intramuscular injection of S-ketamine (Ketanest® S, 10 mg·kg−1, Parke-Davis, Berlin, Freiburg, Germany) and midazolam (Dormicum®, 0.3 mg·kg−1, Hoffmann-La Roche AG, Grenzach-Wyhlen, Germany). Endotracheal intubation was performed with a 9.0 tracheal tube (Rüsch, Rüschelit®, Teleflex Medical GmbH, Kernen, Germany) to enable mechanical ventilation (pressure controlled ventilation, tidal volume 10 mL·kg−1 adapted to expiratory carbon dioxide partial pressure of 35±5 mmHg). Anesthesia was maintained by inhalational isoflurane (targeted expiratory concentration 1.2%; Forene®; AbbVie Deutschland GmbH & Co. KG, Wiesbaden, Germany) and continued throughout the complete experiment. After induction of anesthesia, animals were kept anesthetized until the end of the study.
Cardiovascular instrumentation
All of the following catheterizations and surgical procedures were performed under sterile conditions and after ascertaining an appropriate level of general anesthesia. After placing a quad-lumen central venous catheter into the right jugular vein (6 Fr. Quadlumen Catheter Set, PVB Medizintechnik GmbH, Kirchseeon, Germany) in Seldinger’s technique, anesthesia was supplemented with ketamine (1 mg·kg−1·h−1), midazolam (0.3 mg·kg−1·h−1) and lidocaine (1.5 mg·kg−1·h−1) during the further instrumentation (11). A pulse contour cardiac output catheter was placed in the right femoral artery (5 Fr. PiCCO catheter, Pulsion Medical Systems, Munich, Germany) and a Foley catheter (12 Fr. urinary catheter, Porgès S.A., Le Plessis Robinson-Cedex, France) was inserted to measure urinary output. After the instrumentation, the intravascular catheters were connected to a transpulmonary thermodilution and pulse contour cardiac output computer (PiCCO2, Pulsion Medical Systems, Munich, Germany) to provide continuous hemodynamic surveillance.
Surgical preparation
All animals underwent a median laparotomy. The cecum was detected and incised in order to withdraw 1.5 g·kg−1 feces, while omitting a contamination of the peritoneal cavity. Afterwards this incision was carefully sutured, and the surface of the cecum cleaned and decontaminated. Two 16 Fr. drains were placed between the mesentery of the small intestine and the abdominal wall was closed layer by layer with continuous sutures.
Experimental protocol
The postoperative, healthy baseline measurement (BL) was performed when the following conditions were fulfilled and maintained for one hour (see “Measurements” for details):
- Heart rate (HR) <100 beats per minute (bpm);
- Mean arterial pressure (MAP) ≥70 mmHg;
- Cardiac index (CI): ≥2.5 L·min−1·m−2;
- Arterial lactate ≤1.2 mmol·L−1;
- Blood temperature 38.0−39.8 °C.
Randomization
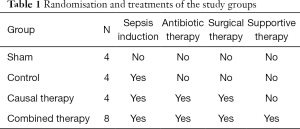
After BL measurement, sheep were randomized to the four study groups using a computer-based algorithm in a 1:1:1:2 ratio (sham, control, causal therapy, combined therapy; see Table 1). Following randomization, the feces was injected into the peritoneal cavity via the indwelling drain in order to induce peritoneal sepsis in all sheep except for the sham group. Basal fluid requirements were substituted in all groups by intravenous infusion of 2 mL·kg−1·h−1 of balanced crystalloid solution (Sterofundin® ISO, B. Braun Melsungen AG, Melsungen, Germany).
Full table
The shock time point was defined based on the Surviving Sepsis Campaign Guidelines 2008 by meeting the following conditions:
- Time from injection of feces ≥4 hours;
- Arterial lactate ≥1.8 mmol·L−1 [i.e., 1.5 times the upper normal limit of sheep (12)];
- MAP ≤60 mmHg.
Surgical and antimicrobial therapy
After shock time measurement (see “Measurements” for details), the animals of the causal and combined therapy groups received peritoneal lavage and antimicrobial chemotherapy. Lavage was performed once after shock time by fractional instillation of four liters of warm saline (38° Celsius) through the abdominal drains until no macroscopic fecal contamination was detectable in the effluent secretion.
Concurrently, intravenous antimicrobial chemotherapy was initiated by bolus injection of 20 mg·kg−1 meropenem (Meronem®, AstraZeneca GmbH, Wedel, Germany), followed by continuous intravenous infusion with 2.5 mg·kg−1·h−1. The sham and control groups received neither of the above-mentioned treatments (see Table 1).
Supportive hemodynamic therapy
Supportive hemodynamic treatment including fluid resuscitation was only performed in the combined therapy group (see Table 1). A balanced crystalloid solution (Sterofundin® ISO, B. Braun Melsungen AG, Melsungen, Germany) and 6% hydroxyethyl starch (HES) 130/0.4 (Volulyte®, Fresenius Kabi Deutschland, Bad Homburg, Germany) were used for fluid resuscitation. Crystalloids and colloids were infused alternately with boluses of 250 mL HES or 500 mL crystalloid. HES was given up to a cumulative maximum dose of 50 mL·kg−1 over the whole interventional period. Afterwards, fluid resuscitation was continued with crystalloids only. Indications for fluid resuscitation were each of the following:
- Global end-diastolic volume index (GEDI) <620 mL·m−2 or below BL value;
- Stroke volume variation (SVV) >13%;
- Hemoglobin (Hb) below BL value.
Fluid boluses were administered until all three conditions were met. Hemoglobin and GEDI were measured every 30 minutes, while SVV was measured continuously. Re-calibration of the PiCCO system was performed every 30 minutes. Norepinephrine infusion was initiated after shock time and titrated continuously to maintain a MAP ≥65 mmHg up to a maximum dose of 5 µg·kg−1·h−1. Furthermore, dobutamine was used up to a maximum dose of 10 µg·kg−1·h−1 if cardiac function index (CFI) was <4.5·min−1. The maximum dosages were chosen from clinical experience, since no further vasoconstrictive or inotropic action could be expected with higher dosages due to tachyphylactic effects.
Measurements
All hemodynamic measurements were obtained in anesthetized animals. Hemodynamic parameters, urinary output as well as arterial and central-venous blood gas analyses were documented at BL, shock time and hourly thereafter. The study animals were monitored until death in deep anesthesia.
Laboratory measurements
Blood and urine samples were taken at BL, shock time and every 4 hours thereafter. The samples were immediately centrifuged and stored at −70 °C for later analysis.
The following variables were determined from the blood and urine samples, respectively:
- Blood gas analyses (electrolytes, oxygen- and carbon dioxide partial pressure, pH, BE, hemoglobin, oxygen saturation, lactate, glucose);
- Parameters of organ (dys-) function (bilirubin, creatinine, creatinine clearance).
End of protocol
Animals surviving the interventional period (8 hours after shock time) were killed with a bolus injection of 100 mL of 1-molar potassium chloride solution after anesthesia was deepened with propofol (4 mg·kg−1).
Outcome variables
Primary outcome measure was survival of the study animals over the interventional period. Secondary outcome measures included hemodynamic variables as well as diuresis and laboratory markers of organ function.
Statistical analysis
Statistical analysis was performed with IBM SPSS statistics software version 24 (IBM, Armonk, New York, United States). Due to the lack of pre-published data on the effects of supportive hemodynamic therapy versus no hemodynamic therapy (e.g., on survival), a rational a priori sample size analysis was not suitable. Thus, all analyses were explorative. All data are presented as median and interquartile range (IQR). Comparisons between groups were made using Mann-Whitney U test or Kruskal-Wallis test dependent on the number of groups to compare. If necessary, post-hoc comparisons were conducted using Dunn’s test. Comparisons between time points were made using Wilcoxon signed-rank test. Asymptotic two-sided P smaller than 0.05 were assumed as statistically relevant differences.
Results
Effects of the instrumentation and laparotomy
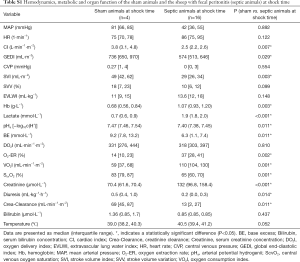
The animals of the sham group showed no signs of systemic inflammation (i.e., increase in heart rate, temperature, or lactate) between BL and shock time and during the following 8 hours (see Table S1). All other measured variables were within physiological ranges according to reference values for anesthetized sheep (12).
Full table
Effects of sepsis induction
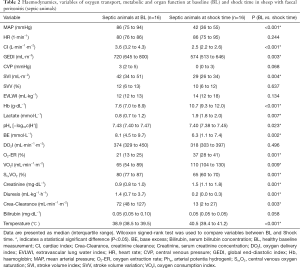
In median sheep developed septic shock after 4.0 hours (IQR 4.0 to 6.8). The effects of sepsis induction are described for animals of the control group between BL and shock time. All septic animals of the other study groups showed similar effects from BL to shock time (see Table 2).
Full table
After feces instillation, the animals developed signs of systemic inflammation and capillary leakage. Core body temperature and hemoglobin concentration increased significantly between BL and shock time (see Table 2).
Between BL and shock time, the animals developed a hypodynamic and hypotensive macrocirculation, as measured by a significant decrease in MAP and CI (see Table 2). Further hemodynamic parameters, such as an increase in heart rate and a decrease of GEDI and SVI confirmed the development of hypovolemia (see Table 2).
Urinary output of all septic animals decreased between BL and shock time, while creatinine concentration increased in the same period, according to a decrease in creatinine clearance (see Table 2 and Figure 1).
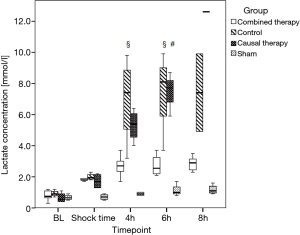
Effects of sepsis treatment
After initiation of the individual group-specific therapy, the animals of the combined therapy group showed signs of improved organ function as compared to the other groups (see details below).
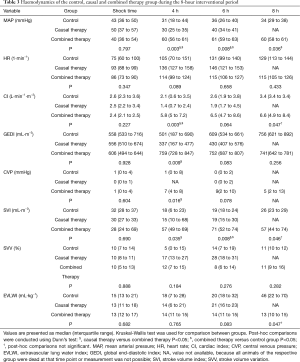
MAP, SVI, CI and GEDI were increased in the combined therapy group as compared to causal and control group (each P<0.05, Table 3). Hemoglobin levels and lactate concentrations were lower in the combined therapy group (each P<0.05, see Figure 1 and Table 4).
Full table
Full table
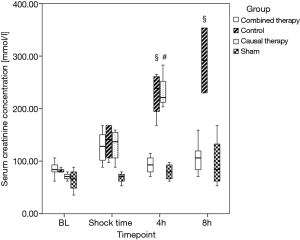
Urinary output was higher in the combined therapy group as compared to the other septic groups, in which anuria developed after shock time (see Table 4). Serum creatinine concentration was significantly lower and creatinine clearance significantly increased in the combined therapy group as compared to the causal and control group (each P<0.05, see Figure 2 and Table 4).
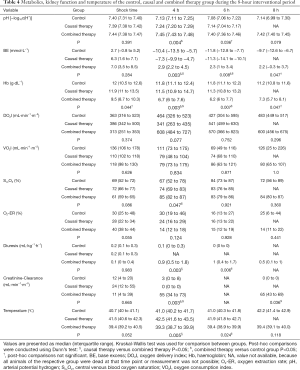
Base excess of the combined therapy animals remained in near physiologic ranges, while the animals of the control and causal therapy group showed a significant decrease in BE during the same time (up to −11.8 mmol·L−1, P<0.05). Accordingly, pH was significantly decreased in these groups (see Table 4).
The variables of oxygen transport (i.e., oxygen delivery index DO2I, oxygen extraction rate O2-ER, oxygen consumption index VO2I) showed no differences between the groups except for a higher central venous oxygen saturation (ScvO2) in the combined therapy group as compared to the control group (see Table 4).
There were no differences in hemodynamic or laboratory variables between the animals of the control group and those of the causal therapy group at any time during the study.
Relative organ weights of the animals were comparable between groups except for the weight of the lungs. The right lung was heaviest in the control group [7.0 g·kg−1 (IQR 6.8 to 7.3)] with significant difference to the sham group [4.8 (IQR 4.1 to 5.6), P<0.05]. Animals of the causal group had the heaviest left lungs [5.7 (IQR 4.8 to 6.3)] with significant difference to the sham group [3.8 (3.7, 4.2), P<0.05, see Table S2].
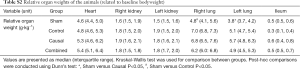
Full table
Fluid balance
The animals of the combined therapy group received 5,500 mL (IQR 4,125 to 7,750) study fluids in the 8-hour interventional period. Cumulative basal fluid administration (2 mL kg−1 h−1) was as follows: combined therapy group 616 mL (IQR 552 to 688), causal group 547 mL (IQR 474 to 574), control group 605 mL (IQR 476 to 640) and sham group 688 mL (IQR 646 to 704). Total fluid balance after 8 hours for the respective groups were as follows: combined therapy group 5,737 mL (IQR 4,414 to 7,945), causal group 532 mL (IQR 465 to 555), control group 580 mL IQR (460 to 622) and sham group 428 mL (IQR 358 to 489) (see Table S3).

Full table
Survival
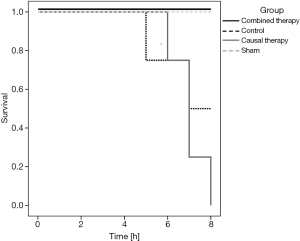
All animals of the combined therapy group and all sham animals survived the 8-hour interventional period. Among the animals of the control group, 50% survived during the same time. None of the causal therapy animals survived the 8-hour interventional period (P<0.001 vs. combined therapy group, P<0.05 vs. control group, P<0.05 vs. sham group, see Figure 3).
Discussion
The present study investigated the effects of causal and supportive hemodynamic treatment strategies in an innovative and clinically relevant model of abdominal septic shock in sheep. All animals except for the sham group developed lactic acidosis and organ dysfunction, indicating that the induction of peritoneal sepsis results in severe systemic inflammation. Treatment of the animals following a study protocol with causal and supportive hemodynamic therapy improved macrohemodynamics, organ function and survival of the affected animals significantly. Notably, causal therapy without supportive hemodynamic treatment did not result in the abovementioned beneficial effects and even worsened survival. This suggests that causal therapy without hemodynamic support worsens outcome more than the natural evolution of sepsis at least in the present model.
The animals in the present investigation met criteria for sepsis-associated organ dysfunction by arterial hypotension, lactic acidosis and an increase in creatinine (13). This means in consequence, that the present model is able to induce sepsis-related organ dysfunction comparable to a clinical setting.
Since the animals of the sham group did not develop signs of systemic inflammation, one can assume that the instrumentation and surgery of the ewes did not induce a pathologic state per se. After peritoneal feces injection, systemic hemodynamics developed to septic shock as in human beings. Hemoconcentration, tachycardia, arterial hypotension and low cardiac output suggest vasodilation and capillary leakage as a central feature. After induction of supportive hemodynamic therapy including fluid resuscitation and vasopressors, a typical hyperdynamic vasodilatory shock developed. Notably, relatively high doses of norepinephrine were necessary to counteract arterial hypotension, which may reflect the severity of shock and the catecholamine hyposensitivity often observed in the clinical setting (14). The significant increase of hemodynamic parameters like CI, GEDI and SVI indicated adequate filling. Furthermore, the mentioned parameters even exceeded BL values (see Table 3), which confirms the development of a hyperdynamic circulatory state which is common in early septic shock (15).
Hemodynamics in the causal therapy group did not improve over time and showed no differences to the animals of the control group (see Table 3). The potential beneficial effects of the causal treatment (i.e., reduced number of bacteria and toxins) may need some minimum time to occur. It seems to be logical, that this time must be bridged by adequate supportive hemodynamic therapy to allow the organism to survive until antimicrobial drugs show their effects. Interestingly, none of the causal therapy animals survived the interventional period, while 50% of the control group animals survived the same time. One probable explanation for this observation may be that peritoneal lavage inevitably involves manipulation of the septic focus, and might thereby worsen systemic inflammation by additional release of toxins into the bloodstream (16,17). This effect may even have been enhanced by the release of toxins due to the bactericidal effects of meropenem with consecutive lysis of bacterial cell membranes (18) and histamine liberation. Especially in Gram-negative sepsis, a 3- to 20-fold increase of endotoxin concentration due to bacteriolysis following application of antibiotic medication could be measured (19). Fekade et al. proved, that the application of anti tumor necrosis factor alpha—antibodies decreases the release of interleukin-6 and -8 (20) and therefore lessens the effect of the “Jarisch-Herxheimer-reaction” including fever and hypotension. Another important reason for the worst outcome of the causal-group animals might be the effect of the warm water for peritoneal lavage, which may cause hypotension and worsening of vasodilatory shock. Mechanisms will most likely be liberation of damage- and pathogen-associated molecular patterns (DAMPs and PAMPs) as well as local vasodilation. In the full therapy group, these effects were offset by supportive hemodynamic therapy. These findings indicate that initiation of causal therapy without adequate hemodynamic support may result in decreased survival in septic patients, a situation which may occur especially in regions with resource-limited settings. Amir and colleagues investigated the effects of the World Health Organization's Integrated Management of Adolescent and Adult Illness (IMAI) in patients with severe infection. Interestingly, the investigators found no difference in survival and organ function (despite lactate clearance) between the patients who received fluid resuscitation according to the IMAI guidelines (n=28) and those who did not (n=94). The administered doses of fluids after 6 hours were 3 L in the IMAI group vs. 1.5 L in the no-IMAI group (21). It must be noted, that the rate of HIV-positive patients in the treated groups were quite high (62%) and no details regarding causal therapy was presented by the authors. Though the findings from the present must be transferred with caution, it should be noted that causal sepsis therapy without fluid resuscitation might be harmful and that potential sources of sepsis, ethnological differences and potential comorbidities must be taken into consideration.
The comparison of causal therapy only and causal plus supportive therapy in the present study showed clearly, that a causal therapy with antibiotics and peritoneal lavage without supportive hemodynamic treatment is not able to improve hemodynamics and organ function in ewes with abdominal sepsis. Causal and supportive therapies have been cornerstones in sepsis therapy for decades. There is good evidence that a delay in causal therapy worsens outcome in patients with septic shock (6). Recent investigations described a potential benefit for septic shock patients receiving restrictive fluid therapy (22) and the debate about restrictive versus liberal resuscitation strategies is still going on. According to the authors of the CLASSIC-trial, restrictive fluid resuscitation might be superior to standard resuscitation protocols in septic patients (22). This raises the question, that if “less therapy” is better than “more therapy”, it might be best to do “no therapy”. Considering the causal therapy group as an “ultra-restrictive” fluid resuscitation regimen (basal requirements with 2 mL·kg−1·h−1 of balanced crystalloid solution), the data from the present study suggest that supportive hemodynamic therapy must include at least some amount of fluids in combination with causal therapy in order to avoid adverse outcome. In the present study, the absence of supportive therapy worsened organ function, hemodynamics and survival in the animals with abdominal sepsis Though the importance of causal therapy is out of the question regarding success of sepsis therapy, the survival of the animals who received causal therapy alone was even worse than in the control group. This may lead to the assumption, that causal and supportive therapy should be initiated together in order to buffer hemodynamic impairment and provide time for the causal therapy to work. This is of utmost importance especially for regions with resource-limited settings and should be considered in recommendations for sepsis therapy within these regions.
Limitations
There are some limitations regarding the present study that should be mentioned. Since this is a model in sheep, the results of this and similar studies should be transferred to human medicine with caution. Though especially sheep models show similar hemodynamic development compared to human beings, results from animal models often differ from clinical trial data for a variety of reasons (3,23). Moreover, the impact of sole hemodynamic without causal therapy was not investigated and may be a focus of future studies. However, since the benefit of causal therapy is clear from clinical data, we did not see a high clinical relevance from a study group of only supportive hemodynamic therapy. Organ function and injury were not investigated in detail in the present study and should be focused in future experiments. The sample size of each group is a limitation, though the results are clearly discriminating and significant.” Another limitation of the present investigation was the use of HES in septic shock, which was an accepted strategy at the time of initiation of the study. The recommended dose of 50 mL·kg−1 BW HES was used in this study. However, since the dose was not adjusted to the relative low bodyweight of the animals, the applied dose of HES was relatively high. We assume that the possible adverse effects of HES on organ function might be negligible after a period of 8 hours. Since the present investigation was a pilot trial, no biometric calculation of the sample size was performed. Due to the low sample size and the reduced number of animals in the control group at 8 hours after shock time, the measured differences should be interpreted with caution. The study investigates intra-abdominal infection and it should be mentioned that the results might be different for other sources of sepsis (e.g., pneumonia). The use of meropenem might miss some Gram-positive bacteria, so in future studies the additional use of antimicrobial drugs with gram positive activity should be considered.
Conclusions
In the present severe model of septic shock with lactic acidosis and organ failure, the initiation of causal therapy including antibiotics and peritoneal lavage without hemodynamic support worsened outcome and organ failure as compared to combined causal and supportive hemodynamic therapy and even natural evolution of sepsis without therapy. In conclusion, the presented investigation underlines the assumption that causal therapy without hemodynamic support might be harmful, and early supportive fluid and vasopressor therapy in septic shock is an essential part of initial sepsis therapy.
Acknowledgements
We acknowledge support by Open Access Publication Fund of University of Muenster.
Funding: The present work was supported by intramural funding of the Department of Anesthesiology, Intensive Care and Pain Medicine, University Hospital of Muenster, Muenster, Germany.
Footnote
Conflicts of Interest: TG Kampmeier received travel reimbursements and honoraria as a consultant from Fresenius Kabi Germany. M Westphal is currently Chief Medical Officer of Fresenius Kabi Germany. S Rehberg has received travel fees provided by Orion Pharma and Amomed Pharma and is Medical Advisor for Fresenius Kabi Germany and Amomed Pharma. Other authors have no conflicts of interest to declare.
Ethical Statement: The present study was approved by the Animal Care Committee of the State Government of North-Rhine Westphalia (LANUV NRW, Recklinghausen, Germany) with the approval no. 84-02.04.2011.A300.
References
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369:840-51. [Crossref] [PubMed]
- Fleischmann C, Thomas-Rueddel DO, Hartmann M, et al. Hospital Incidence and Mortality Rates of Sepsis. Dtsch Arztebl Int 2016;113:159-66. [PubMed]
- Kampmeier TG, Ertmer C, Rehberg S. Translational research in sepsis - an ultimate challenge? Exp Transl Stroke Med 2011;3:14. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589-96. [Crossref] [PubMed]
- Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med 2014;42:2409-17. [Crossref] [PubMed]
- Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 2011;39:2066-71. [Crossref] [PubMed]
- Seymour CW, Gesten F, Prescott HC, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med 2017;376:2235-44. [Crossref] [PubMed]
- Byrne L, Van Haren F. Fluid resuscitation in human sepsis: Time to rewrite history? Ann Intensive Care 2017;7:4. [Crossref] [PubMed]
- Weibel S, Jokinen J, Pace NL, et al. Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. Br J Anaesth 2016;116:770-83. [Crossref] [PubMed]
- Kampmeier T, Arnemann P, Heßler M, et al. Provision of physiologic data and reference values in awake and anaesthetized female sheep aged 6-12 months. Vet Anaesth Analg 2017;44:518-28. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Andreis DT, Singer M. Catecholamines for inflammatory shock: a Jekyll-and-Hyde conundrum. Intensive Care Med 2016;42:1387-97. [Crossref] [PubMed]
- Yang S, Cioffi WG, Bland KI, et al. Differential alterations in systemic and regional oxygen delivery and consumption during the early and late stages of sepsis. J Trauma 1999;47:706-12. [Crossref] [PubMed]
- Prins JM, van Agtmael MA, Kuijper EJ, et al. Antibiotic-induced endotoxin release in patients with gram-negative urosepsis: a double-blind study comparing imipenem and ceftazidime. J Infect Dis 1995;172:886-91. [Crossref] [PubMed]
- Lepper PM, Held TK, Schneider EM, et al. Clinical implications of antibiotic-induced endotoxin release in septic shock. Intensive Care Med 2002;28:824-33. [Crossref] [PubMed]
- Crosby HA, Bion JF, Penn CW, et al. Antibiotic-induced release of endotoxin from bacteria in vitro. J Med Microbiol 1994;40:23-30. [Crossref] [PubMed]
- Hurley JC. Antibiotic-induced release of endotoxin: a reappraisal. Clin Infect Dis 1992;15:840-54. [Crossref] [PubMed]
- Fekade D, Knox K, Hussein K, et al. Prevention of Jarisch-Herxheimer reactions by treatment with antibodies against tumor necrosis factor alpha. N Engl J Med 1996;335:311-5. [Crossref] [PubMed]
- Amir A, Saulters KJ, Muhindo R, et al. Outcomes of patients with severe infection in Uganda according to adherence to the World Health Organization's Integrated Management of Adolescent and Adult Illness fluid resuscitation guidelines. J Crit Care 2017;41:24-8. [Crossref] [PubMed]
- Hjortrup PB, Haase N, Bundgaard H, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med 2016;42:1695-705. [Crossref] [PubMed]
- Poli-de-Figueiredo LF, Garrido AG, Nakagawa N, et al. Experimental models of sepsis and their clinical relevance. Shock 2008;30 Suppl 1:53-9. [Crossref] [PubMed]