Limited resection in clinical stage I non-small cell lung cancer patients aged 75 years old or more: a meta-analysis
Introduction
Lung cancer is the leading cause of cancer-related death around the world (1). With the development of medical management, the number of elderly patients with non-small cell lung cancer (NSCLC) is rapidly increasing. As reported, more than 40% of those diagnosed with lung cancer are over 75 years old (2). Patients over 75 years old usually represent a very heterogeneous group. They usually had shorter life expectancy and multiple comorbidities, but presented with different functional performance, nutritional status, and social resources. It is concluded that therapeutic strategy might be different from that of younger patients (3,4).
The best treatment for early stage NSCLC patients is anatomic lobectomy with mediastinal lymph node dissection (5). However, a considerable number of aged patients with early stage NSCLC might be unable to tolerate lobectomy due to their poor cardiopulmonary function and complicated comorbidities. Previous studies have shown complicated results in defining favorable surgical procedure in aged patients (6-13).
Proponents hold the opinion that limited resection should be indicated if the patient had an increased risk of complications as limited resection could provide similar survival rate compared with lobectomy (8-11). The reduction in morbidity and mortality (14) provided by limited resection might benefit the aged given their reduced pulmonary function reserve, associated comorbidities, and higher propensity for surgical complications. While opponents argued that there were poorer survival (12,13) after limited resection when compared with lobectomy.
Given that controversy remained as to whether limited resection could be a reasonable alternative to lobectomy in the treatment of early stage NSCLC for aged patients, and no randomized trials have been published, we conducted this analysis to examine the outcome of morbidity and prognosis after limited resection compared with lobectomy in clinical stage I NSCLC patients aged over 75 years old.
Methods
Search strategy and selection criteria
A systemic search of database including PubMed, OVID and Cochrane (Cochrane Database of Systematic Reviews, American College of Physicians Journal Club, ACP Journal Club, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Health Technology Assessment, and NHS Economic Evaluation Database) was carried out to identify the potential relevant studies published, with the search terms of ‘lung cancer’; ‘lung neoplasm’; ‘lobectomy’; ‘limited resection’; ‘sublobectomy’; ‘sublobe resection’; ‘limited resection’; ‘wedge resection’; ‘segmentectomy’; ‘aged’; ‘elderly’; and ‘octogenarian’. In this study, only studies compared postoperative outcome of clinical stage I NSCLC patients aged ≥75 years between limited resection and lobectomy were included. Only articles published in English were included. Case reports, review articles, letters, editorials, and expert opinions without original data were excluded. Duplicate publications were excluded if same results were reported. AJCC lung cancer staging 7th edition was used in all studies.
Data extraction and quality control
Titles and abstracts from electronic searches and potential relevant full papers were selected by two reviewers (Z Zhang and H Fang) independently. Studies were enrolled after the full articles have been assessed by two reviewers. Disagreement between the two reviewers was settled by discussing with a third reviewer (D Liu). Newcastle-Ottawa Scale (NOS) (15) criteria was used in assessing the quality and bias of included non-randomized studies. Selection of study groups, comparability of the groups and ascertainment of either the exposure or outcomes of interest were assessed for the quality of each study.
Data including sample size, clinical stage, perioperative morbidity and mortality, total recurrence, distant recurrence, local-regional recurrence, overall survival and lung cancer specific survival in each study were extracted.
Statistical analysis
Data extracted were analyzed with Revman 5.1. Risk ratios (RRs) or hazards ratios (HRs) were used in calculating dichotomous variables. Cochrane’s Q and I2 statistics were used in heterogeneity analysis. Fixed-effects model was used when there was minor or no heterogeneity between studies (P>0.10, or P≤0.10 but I2≤50%). Otherwise, random-effects model was accepted when heterogeneity existed. Two-tailed P value
Results
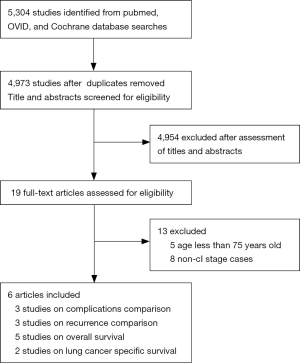
The results of the systemic search were outlined in Figure 1. 5,304 citations were identified electronically, 3,331 by searching PubMed, 1,692 by searching the OVID, 281 by searching the Cochrane Central Register of Controlled Trials. After review of all titles and abstracts, 19 papers were selected for full text review. Eight studies were excluded as non-clinical stage I NSCLC patients were included, or the inclusion stage criteria was not clarified in the article. Five studies were excluded as younger patients (8-13) were included. Of included studies, perioperative complications were compared in 3 studies (9-11). Overall survival (OS), lung cancer specific survival (LCSS) and recurrence were compared in 5 (8-12), 2 (12,13), and 3 (9-11) studies respectively. 2 articles (9,12) compared outcome between segmentectomy and lobectomy. 1 article compared outcome between wedge resection and lobectomy (13). Others did not specify surgical procedure of limited resection (8,10,11). A total of 3,461 patients were included, of whom 1,323 patients received limited resection and 2,139 patients received lobectomy. Baseline characteristics of these patients are listed in Table 1.
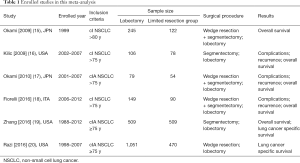
Full table
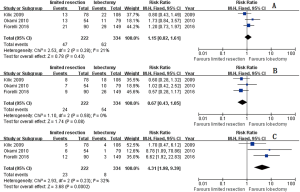
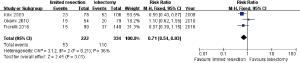
Three studies (9-11) including 222 limited resections and 334 lobectomies reported their results of postoperative complications and mortalities. The pooled postoperative complication ratios are 23.87% and 32.93% in limited resection and lobectomy group respectively (risk ratio RR =0.71; 95% CI, 0.54–0.93; P=0.01; I2=48%, P=0.15) (Figure 2).
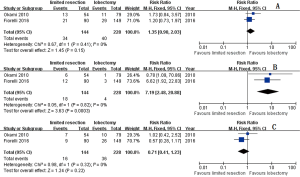
The pooled total recurrence ratio, distant recurrence ratio, and local-regional recurrence ratio were 21.17%, 10.81%, and 10.36% respectively in limited resection group (9-11). Similar total recurrence ratio (18.56%, RR =1.15; 95% CI, 0.82–1.61; P=0.43; I2=21%, P=0.28), and distant recurrence ratio (16.17%, RR =0.67; 95% CI, 0.43–1.05; P=0.08; I2=0%, P=0.58) were observed in lobectomy group. Lower local-regional recurrence ratio (2.40%, RR =4.31; 95% CI, 1.98–9.39; P2=32%, P=0.23) were observed in lobectomy group (Figure 3).
OS were reported by five studies (8-12) including 1,941 patients, of whom 1,060 received limited resection and 881 received lobectomy. Compared with lobectomy, patients received limited resection got poorer OS (hazard ratio HR =1.24; 95% CI, 1.07–1.44; P=0.004) without significant heterogeneity (P=0.35; I2=9%). LCSS were reported by 2 studies (12,13). Pooled results showed poorer LCSS in limited resection group compared with that in lobectomy group (HR =1.37; 95% CI, 1.14–1.64; P2=0%, P=0.60) (Figure 4).
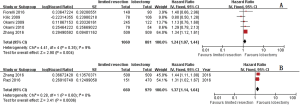
Three studies (8,10,11) mixed two limited resection procedure (segmentectomy and wedge resection) in their study. Pooled total recurrent ratio, distant recurrent ratio, and local-regional recurrent ratio were 23.61%, 11.11%, and 12.50% respectively in mixed limited resection group. Similar total recurrent ratio (17.54%, RR =1.35; 95% CI, 0.90–2.03; P=0.15; I2=0%, P=0.41), and distant recurrent ratio (15.79%, RR =0.71; 95% CI, 0.41–1.23; P=0.22; I2=0%, P=0.32) were observed in lobectomy group without heterogeneity. Lower local-regional recurrent ratio (19.30%, RR =7.19; 95% CI, 2.48–20.88; P2=0%, P=0.82) was observed in lobectomy group. And similar OS was shown between groups (HR =1.22; 95% CI, 0.91–1.62; P =0.18; I2=0%, P=0.85) (Figures 5,6).

Two studies compared outcome between segmentectomy and lobectomy (9,12). One compared perioperative complications, recurrence, and survival results between groups (9), and OS and LCSS were compared in the other study (12). In Kilic and colleagues’ study (9), 78 stage I NSCLC aged patients received segmentectomy, 106 received lobectomy. Compared with lobectomy, there was fewer complications (11.5% vs. 25.5%, P=0.02), similar 5-year disease-free survival (49.8% vs. 45.5%, P=0.80) and OS (46% vs. 47%, P=0.28) in segmentectomy group. In Zhang and colleagues’ study (12), patients treated with segmentectomy had significantly poorer OS (HR =1.239, 95% CI, 1.093–1.405, P=0.001) and LCSS (HR =1.308, 95% CI, 1.094–1.563, P=0.003). After pooled the OS outcome, poorer OS was shown in limited resection group when compared with that in lobectomy group (HR =1.25; 95% CI, 1.06–1.49; P=0.01; I2=75%, P=0.04) (Figure 7).
Funnel plots did not show any publication bias based on the data of morbidity, recurrence, and overall survival (Figure 8).

Discussion
In this article, we focused on perioperative and long-term efficacy of limited resection in aged patients over 75 years old. As the outcome demonstrated, though less perioperative complications occurred after limited resection, inferior local-regional recurrence, OS and LCSS were shown after limited resection.
Lung cancer is the leading cause of cancer-related death in aged patients. More than 40% of pathological diagnosed lung cancer are over 75 years old (2). The intrinsic feature of aged patients such as higher incidence of comorbidities, impaired cardiopulmonary function and limited life expectancy (16,17) have occluded many of them from lung surgery though pulmonary resection remained to be the best treatment for aged patients with early stage NSCLC.
Some studies (18,19) have shown that lung cancer surgery might result in poor survival outcome in aged patients. After analyzing 12,439 postsurgical lung cancer patients, Romano and colleagues (18) found that the risk of death in patients aged over 79 years old was three times higher than that of younger patients. Similar outcomes have been demonstrated by the ITACARE working group (19).
Anderson and colleagues’ study has shown that though people in their 80 s have a 50% chance of living 5 more years, an average life expectancy of 1.5-year old could be observed in patients with untreated early-stage NSCLC (20). With the development of perioperative management, minimally invasive surgery and fast recovery, significant reduction in perioperative mortality, morbidity and improved long-term survival rates have been achieved. Recently published studies have shown excellent short and long-term outcome after pulmonary resection in aged patients (9-13). Furthermore, though aged patients undergoing lung resection have higher incidence of morbidity and mortality compared (2), the inferiority might be offset by the lower recurrence ratio (21).
As demonstrated by Lung Cancer Study Group in 1995, three times higher cancer recurrence ratio was observed after limited resection, lobectomy with lymph node dissection was the standard surgical procedure for early stage lung cancer (5). However, the elderly subgroup patients were not further analyzed in this randomized clinical trial.
As published evidence showed an improved postoperative morbidity and mortality (22), and preservation of pulmonary function (23) after limited resection, surgical procedure (limited resection or lobectomy) should be carefully selected to balance the risk of postoperative morbidity and mortality against the risk of cancer related survival. These safety and functional benefit in postoperative morbidity, mortality and pulmonary preservation after limited resection have been verified in our study. After analyzing 222 limited resection and 334 lobectomies in aged patients, significant lower postoperative morbidity and mortality after limited resection was found when compared with lobectomy. The intrinsic features of having preexisting comorbidities, and impaired pulmonary function of aged patients should be an explanation of the lower postoperative complication in limited resection group.
Recently, Yang and colleagues published a protocol of randomized controlled multicenter non-inferiority trial which compared the long term and short-term outcome including DFS, OS, morbidity and mortality for elderly patients (≥70 years) with early-stage NSCLC (24). This is the first trial tried to find out which procedure (limited resection or lobectomy) should be preferred in aged patients.
The oncologic efficacy of limited resection for aged patients have been debated by decades. Proponents of limited resection hold the opinion that the recurrence and survival difference between patients who have undergone lobectomy and limited resection might vanish in aged patients. Reported evidence have shown that the survival benefit offered by lobectomy over limited resection has gradually decreased over the past 2 decades (6). Another large retrospective study (25) comparing long term outcome after limited resection and lobectomy in 1,272 stage I NSCLC showed comparable 5-year cancer specific survival between groups (92.4% vs. 85.7%, P=0.77). Several other retrospective studies with relatively small numbers also suggested similar survival outcome following limited resection compared with lobectomy (26).
Different outcomes have been reported by several other studies (12,13,27,28). After analyzing a total of 1018 patients, an OS (HR =1.343, 95% CI, 1.117–1.613, P=0.002) and LCSS (HR =1.443, 95% CI, 1.106–1.884, P=0.007) benefit of lobectomy over limited resection (segmentectomy only) were observed after propensity score matching (12). Another study (13) showed that after long term follow up (43 months in limited resection group vs. 60 months in lobectomy group), improved five-year LCSS was observed in lobectomy group when compared with that in limited resection group (64.5% vs. 42.7%, P27,28) also confirmed these inferior survival outcomes after limited resection.
Our results analyzed recurrence ratio, OS and LCSS difference between limited resection and lobectomy groups and confirmed the survival benefit after lobectomy over limited resection. Though similar total and distant recurrence ratio could be observed between groups, there were higher local-regional recurrence ratio (10.36% vs. 2.40%, RR =4.31, P13). Also much more satisfied tumor free margin after lobectomy would be another explanation.
There are several limitations that need to be acknowledged in this study. Retrospective nature which subjects it to the possible selection bias associated with surgical procedure chosen is the major limitation of this analysis. The oncologic efficacy of limited resection in this specific cohort of patients needs to be assessed in prospective, randomized study. Another limitation is that most of included studies amalgamate wedge resection and segmentectomy together, though we performed subgroup analysis between segmentectomy and lobectomy, only two studies could be analyzed. As a result, the robustness of our results could be influenced. Furthermore, data regarding radiological features such as ground glass opacity that represent minimally invasive nature of lung cancer are unavailable among all studies. It might be possible that different outcome could be shown with only pure ground glass nodules being included.
After pooled analysis of six studies, this analysis showed better LCSS, and OS after lobectomy in comparison with limited resection for patients with clinical stage I NSCLC patients aged over 75 years, though perioperative morbidity were higher in lobectomy group. These results confirm that lobectomy should be considered if tolerable in aged patients. These outcomes should be verified in randomized prospective studies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This is a metaanalysis of retrospective study, the ethical statement was waived.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Brown JS, Eraut D, Trask C, et al. Age and the treatment of lung cancer. Thorax 1996;51:564-8. [Crossref] [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [Crossref] [PubMed]
- Aoki T, Tsuchida M, Watanabe T, et al. Surgical strategy for clinical stage I non-small cell lung cancer in octogenarians. Eur. J. Cardiothorac. Surg 2003;23:446-50. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-622; discussion 622-3. [Crossref] [PubMed]
- Yendamuri S, Sharma R, Demmy M, et al. Temporal trends in outcomes following sublobar and lobar resections for small (≤2 cm) non-small cell lung cancers--a Surveillance Epidemiology End Results database analysis. J Surg Res 2013;183:27-32. [Crossref] [PubMed]
- Dominguez-Ventura A, Cassivi SD, Allen MS, et al. Lung cancer in octogenarians: factors affecting long-term survival following resection. Eur J Cardiothorac Surg 2007;32:370-4. [Crossref] [PubMed]
- Okami J, Higashiyama M, Asamura H, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol 2009;4:1247-53. [Crossref] [PubMed]
- Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg 2009;87:1662-6; discussion 1667-8.
- Okami J, Ito Y, Higashiyama M, et al. Sublobar resection provides an equivalent survival after lobectomy in elderly patients with early lung cancer. Ann Thorac Surg 2010;90:1651-6. [Crossref] [PubMed]
- Fiorelli A, Caronia FP, Daddi N, et al. Sublobar resection versus lobectomy for stage I non-small cell lung cancer: an appropriate choice in elderly patients? Surg Today 2016;46:1370-82. [Crossref] [PubMed]
- Zhang Y, Yuan C, Zhang Y, et al. Survival following segmentectomy or lobectomy in elderly patients with early-stage lung cancer. Oncotarget 2016;7:19081-6. [PubMed]
- Razi SS, John MM, Sainathan S, et al. Sublobar resection is equivalent to lobectomy for T1a non-small cell lung cancer in the elderly: a Surveillance, Epidemiology, and End Results database analysis. J Surg Res 2016;200:683-9. [Crossref] [PubMed]
- Smythe WR. Treatment of stage I non-small cell lung carcinoma. Chest 2003;123:181S-7S. [PubMed]
- Wells GA, Shea BJ, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_ epidemiology/oxford.htm
- Castillo MD, Heerdt PM. Pulmonary resection in the elderly. Curr Opin Anaesthesiol 2007;20:4-9. [Crossref] [PubMed]
- Fentiman IS, Tirelli U, Monfardini S, et al. Cancer in the elderly: why so badly treated? Lancet 1990;335:1020-2. [Crossref] [PubMed]
- Romano PS, Mark DH. Patient and hospital characteristics related to in-hospital mortality after lung cancer resection. Chest 1992;101:1332-7. [Crossref] [PubMed]
- Vercelli M, Quaglia A, Casella C, et al. Cancer patient survival in the elderly in Italy. ITACARE Working Group. Tumori 1997;83:490-6. [Crossref] [PubMed]
- Anderson RN. United States Life Tables, 1997. Natl Vital Stat Rep 1999;47:1-37. [PubMed]
- Donington J, Ferguson M, Mazzone P, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small-cell lung cancer. Chest 2012;142:1620-35. [Crossref] [PubMed]
- Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg 1997;113:691-8. [Crossref] [PubMed]
- Pastorino U, Valente M, Bedini V, et al. Limited resection for Stage I lung cancer. Eur J Surg Oncol 1991;17:42-6. [PubMed]
- Yang F, Sui X, Chen X, et al. Sublobar resection versus lobectomy in Surgical Treatment of Elderly Patients with early-stage non-small cell lung cancer (STEPS): study protocol for a randomized controlled trial. Trials 2016;17:191. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Takami K, et al. Treatment strategy for patients with small peripheral lung lesion(s): intermediate-term results of prospective study. Eur J Cardiothorac Surg 2008;34:1068-74. [Crossref] [PubMed]
- Whitson BA, Groth SS, Andrade RS, et al. Lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943-50. [Crossref] [PubMed]
- Liu Y, Huang C, Liu H, et al. Limited resection versus lobectomy for stage IA (T1a) non-small-cell lung cancer: a meta-analysis study. World J Surg Oncol 2014;12:138. [Crossref] [PubMed]