Effect of erlotinib plus bevacizumab on brain metastases in patients with non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer death worldwide. The recent development of the epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) gefitinib and erlotinib has markedly improved the treatment of advanced non-small cell lung cancer (NSCLC) (1). Brain metastasis (BM) occurs in approximately 20–40% of NSCLC patients (2,3), and is generally the site of metastasis most associated with a poor prognosis (4). Without treatment, the prognosis for NSCLC patients with BM is poor, with a median overall survival (OS) of 1–2 months (5).
BM in NSCLC is typically treated with radiation therapy (RT), including stereotactic radiosurgery (SRS) and whole-brain radiotherapy (WBRT) (6). However, RT is associated with complications such as leukoencephalopathy, meaning that systemic treatment may be preferable (7). Despite this, chemotherapy for BM in NSCLC has traditionally been considered ineffective because of the difficulties in penetrating the blood–brain barrier (8). Moreover, patients with BM were initially excluded from clinical trials for bevacizumab after the occurrence of a fatal cerebral hemorrhage in a phase I study (9). On the other hand, the contraindication of bevacizumab for BM was removed from the EU Summary of Product Characteristics in 2009 after the submission of comprehensive safety data. The BRAIN study demonstrated the efficacy and safety of bevacizumab with first-line paclitaxel and carboplatin therapy in patients with NSCLC and asymptomatic untreated BM (10). At present, there is increasing interest in the systemic treatment of BM in NSCLC patients (6). In particular, good responses to treatment with molecular targeted drugs [such as EGFR-TKIs and anaplastic lymphoma kinase (ALK) inhibitors] have been reported for BM (11,12). Furthermore, it has been shown that the concentration of erlotinib in cerebrospinal fluid may be higher than that of other EGFR-TKIs (such as gefitinib), thus increasing its efficacy in the treatment of BM, especially leptomeningeal metastases (13).
Recently, Seto et al. suggested that combination therapy of erlotinib plus bevacizumab (E+B) was effective and safe in patients with EGFR mutation-positive NSCLC (14), a patient population that is at increased risk of developing BM (15). The successful management of NSCLC patients using E+B therefore represents an effective alternative to RT for BM. In the current report, we review 8 cases treated by E+B as first-line therapy for BM.
Methods
We retrospectively examined data from who had patients with NSCLC and BM, and harbored EGFR mutations, and, who were given E+B as first-line therapy for BM until August 2017 at our institution. Patients receiving local therapy for BM, such as surgery or radiotherapy, were excluded.
Patients were diagnosed with BM at the first medical examination, during postoperative observation after completion resection, or during systemic chemotherapy.
EGFR mutations were diagnosed in surgical specimens in recurrent patients, and by transbronchial biopsy specimen in advanced lung cancer patients. BM was diagnosed by computed tomography (CT) or magnetic resonance imaging (MRI). Extracranial disease was examined by CT or positron emission tomography (PET) at the time of detection of BM. Patients were orally administered erlotinib (once daily at 150 mg/body) plus bevacizumab by intravenous infusion (15 mg/kg on day 1 of a 21- or 28-day cycle). For adverse events related to erlotinib, treatment was suspended until the adverse events resolved, and dose reduction of erlotinib followed. For adverse events related to bevacizumab, treatment was suspended and bevacizumab was retried after resolution of the adverse events. Tumor lesions were assessed radiologically every 1–3 months. Treatment was administered sequentially until disease progression or the occurrence of intolerable adverse events. Where E+B treatment was suspended, some patients then selected systemic chemotherapy (e.g., platinum-based doublet regimens) or local treatment for BM (e.g., SRS or WBRT), while others selected best supportive care. The objective tumor response was evaluated by CT or PET for extracranial disease and by CT or MRI for BM, according to the Response Evaluation Criteria in Solid Tumors guidelines (16).
Survival curves were calculated using the Kaplan-Meier method. Statview version 5.0 (Abacus Concepts, Inc., Berkeley, CA, USA) was used for all statistical analyses.
Results
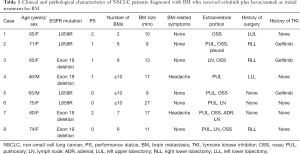
Eight patients with NSCLC who were diagnosed with BM, including two men and six women with a median age of 65 years (range, 46–75 years), received E+B as initial treatment for BM. The patient characteristics are shown in Table 1. Six patients had an Eastern Cooperative Oncology Group performance status (PS) of 0/1, and 2 patients had a PS of 2 at the start of therapy. Four patients had an L858R EGFR mutation, while four had an exon 19 deletion. BMs were variable in number and size (Table 1) and caused symptoms (headache) in 2 patients. Extracerebral metastases were found in the lung in 6 patients, bone in 6 patients, adrenal glands in 1 patient, and lymph nodes in 4 patients. BM was diagnosed in 3 patients during postoperative observation after completion resection, in 2 patients during the first medical examination, and in 3 patients during systemic chemotherapy (including for recurrent disease after surgery). In terms of surgical history, 5 patients had recurrent disease after surgery, and 3 patients had been pretreated with gefitinib before being diagnosed with BM (Figure 1). Regarding the additional brain metastatic sites of the 3 patients previously treated with gefitinib, at the time of initiating treatment with erlotinib plus bevacizumab, case 2 was diagnosed with a new lesion (pulmonary and bone metastasis), case 3 was controlled expect for brain metastatic sites, and case 5 was diagnosed the recurrence of the primary lesion.
Full table
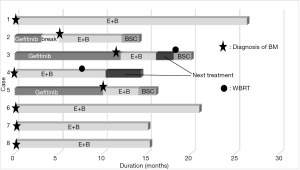
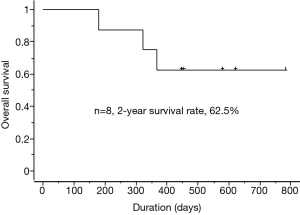
The outcomes of E+B treatment are summarized in Table 2. In terms of the objective tumor response for BM, 7 patients had a partial response (PR) and 1 patient had a complete response. In terms of the objective tumor response for extracerebral metastases, 6 patients had a PR and 1 patient had a complete response. The 2-year survival rate following treatment with E+B was 62.5% (Figure 2). Each treatment course is shown in Figure 1. Three patients who received prior treatment with gefitinib had an E+B treatment duration of less than 1 year. However, the 5 patients who received E+B as first-line treatment had treatment duration of 1 year or longer. All patients had suspended E+B treatment because of adverse events (cases 1–4 and 6–8) or for personal reasons (case 5), although 7 patients had restarted E+B with dose reduction of erlotinib (cases 1–4 and 6–8). One patient could not continue on E+B for personal reasons (case 5). Adverse events of erlotinib treatment included skin and subcutaneous tissue disorders (cases 1–4 and 6–8) and malaise (cases 1, 2, and 7), with other events such as gastrointestinal disorders not observed. Adverse events of bevacizumab were hypertension (case 1) and malaise (case 2), and no critical adverse events such as hemoptysis and proteinuria were observed. Among the 4 patients who discontinued E+B treatment, 2 patients had tumor regrowth (case 3 had regrowth and a new BM lesion, while a new lesion was detected in case 4), 1 patient had malaise (case 2), and 1 patient discontinued for personal reasons (case 5). The patient in case 4 was still alive and undergoing third-line treatment at the time of writing, with a systemic PR and BM controlled using WBRT. The cases 2 and 3 patients died from primary illness after therapy. Two patients (cases 3 and 4) received WBRT, the timing of which is described in Figure 1. Four patients were still receiving treatment without tumor regrowth at the time of writing (cases 1, 6, 7, and 8).

Full table
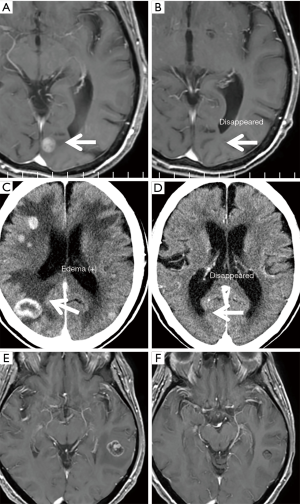
Three representative cases who received E+B treatment for more than 1 year are presented in Figure 3. In case 1, two BMs have virtually disappeared, along with a remarkable effect on bone metastasis (Figure 3A,B). In case 6, more than 10 BMs with edema of various sizes almost disappeared, along with a remarkable reduction of the main tumor and mediastinal lymph nodes (Figure 3C,D). In case 7, seven BMs of various sizes were good PR, and the BM-related symptom of headache improved, along with a remarkable reduction in the size of the primary tumor and metastatic lesions (Figure 3E,F).
Discussion
To our knowledge, our results are notable for two reasons. First, we reviewed cases of NSCLC patients with BM who had not previously received RT such as SRS or WBRT, but instead received E+B as initial treatment for BM. Moreover, this study included patients with BM-related neurologic symptoms and multiple BMs, for which radiotherapy is typically indicated. Second, this eight-case analysis was a retrospective study of consecutive data only from our institution. Although the patient population in our study was small, studies on E+B as initial treatment for BM in a single institution have not previously been reported.
The present study demonstrated three major findings. First, E+B treatment of all eight patients was found to be safe, with no severe complications or progression of BM-related neurologic symptoms, despite existing safety concerns about the use of E+B such as cerebral hemorrhage. However, a limitation of our data is that BM patients with severe brain edema were excluded from the study population. This finding supports the selection of E+B as initial treatment for BM in NSCLC patients with EGFR mutations, given the complication that can arise with RT such as leukoencephalopathy related to activities of daily living (ADL). Second, this study showed the effectiveness of E+B for BM in NSCLC patients with BM-related neurologic symptoms and multiple BMs. All patients had a PR, except for 1 patient that had a complete response, and 4 patients were continuing treatment at the time of writing (cases 1, 6–8). Some previously (10,11) studies have examined the effects of chemotherapy (such as cytotoxic agents or molecular targeted drugs) on BM, although none showed that chemotherapy was effective in all patients with BM. Molecular targeted drugs such as EGFR-TKIs and ALK inhibitors have recently been used to treat BM in NSCLC patients positive for driver mutations (11,12) despite the limitations posed by the blood-brain barrier. However, these previous studies excluded patients with BM-related neurologic symptoms and multiple BMs. Based on our findings, we recommend treatment with E+B for BM in patients with NSCLC and EGFR mutations, including those with BM-related neurologic symptoms and multiple BMs. BM-related neurologic symptoms can lead to a rapid decline in ADL, meaning that rapid, effective treatment, such as E+B, is recommended for BM. Moreover, treatment that reduces ADL, such as WBRT, should be avoided for NSCLC patients with driver mutations and a long-term prognosis. Third, our findings support the use of E+B as first-line therapy for BM in NSCLC patients, as E+B treatment was more effective when used as first-line therapy for BM compared with E+B after previous treatment with EGFR-TKI. Pre-treatment with EGFR-TKI may reduce the effectiveness of E+B treatment because resistance may develop, including T790M mutations in EGFR. Recently developed EGFR-TKI such as osimertinib and AZD 3759 can more readily penetrate the blood-brain than erlotinib can (17). Future studies should therefore examine the effect of adding bevacizumab to novel EGFR-TKI.
This study indicates that E+B may be used as first-line therapy for BM in NSCLC, even in patients with BM-related neurologic symptoms and multiple BMs. In the future, next-generation EGFR-TKI plus bevacizumab may represent a new treatment strategy for BM in NSCLC.
Acknowledgements
We thank Clare Cox, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Ethics Committee of the University of Occupational and Environmental Health Japan approved this study (H26-15). Informed consent was obtained from the patients before the study commenced.
References
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]
- Sørensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol 1988;6:1474-80. [Crossref] [PubMed]
- Mamon HJ, Yeap BY, Jänne PA, et al. High risk of brain metastases in surgically staged IIIA non-small-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J Clin Oncol 2005;23:1530-7. [Crossref] [PubMed]
- Lin JJ, Cardarella S, Lydon CA, et al. Five-Year Survival in EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs. J Thorac Oncol 2016;11:556-65. [Crossref] [PubMed]
- Schettino C, Bareschino MA, Rossi A, et al. Targeting angiogenesis for treatment of NSCLC brain metastases. Curr Cancer Drug Targets 2012;12:289-99. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282-7. [Crossref] [PubMed]
- Zhong X, Huang B, Feng J, et al. Delayed leukoencephalopathy of non-small cell lung cancer patients with brain metastases underwent whole brain radiation therapy. J Neurooncol 2015;125:177-81. [Crossref] [PubMed]
- Cedrych I, Kruczała MA, Walasek T, et al. Systemic treatment of non-small cell lung cancer brain metastases. Contemp Oncol (Pozn) 2016;20:352-7. [Crossref] [PubMed]
- Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol 2001;19:843-50. [Crossref] [PubMed]
- Besse B, Le Moulec S, Mazières J, et al. Bevacizumab in Patients with Nonsquamous Non-Small Cell Lung Cancer and Asymptomatic, Untreated Brain Metastases (BRAIN): A Nonrandomized, Phase II Study. Clin Cancer Res 2015;21:1896-903. [Crossref] [PubMed]
- Namba Y, Kijima T, Yokota S, et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: review of 15 clinical cases. Clin Lung Cancer 2004;6:123-8. [Crossref] [PubMed]
- Muhammet Hacioglu B, Kostek O, Erdogan B, et al. Targeted therapy with anaplastic lymphoma kinase inhibitors in non-small cell lung cancer even with brain metastasis. J BUON 2017;22:586-91. [PubMed]
- Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399-405. [Crossref] [PubMed]
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236-44. Erratum in: Lancet Oncol 2014;15:e475. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Itakura M, et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 2015;20:674-9. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Ahn MJ, Kim DW, Cho BC, et al. Activity and safety of AZD3759 in EGFR-mutant non-small-cell lung cancer with CNS metastases (BLOOM): a phase 1, open-label, dose-escalation and dose-expansion study. Lancet Respir Med 2017;5:891-902. [Crossref] [PubMed]


