Efficacy characteristics of different therapeutic modalities for locally advanced prostate cancer: a Bayesian network meta-analysis of randomized controlled trials
Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed malignancy in elderly men worldwide (1). In recent decades, along with the development of the elevated serum prostate-specific antigen (PSA) level measurement, abnormal digital rectal examination (DRE) finding and transrectal ultrasonography (TRUS), the incidence and detection rate of PCa was continuously increasing. Furthermore, it had become the first malignancy of males and the second leading cause of cancer-specific death in the United States (1,2). Therefore, there is an urgent need to find a better therapeutic method of PCa.
Recently, the progress of various treatment modalities has greatly improved the surgical and oncological outcomes of patients with PCa (3). Currently, radical radiotherapy (RT) or radical prostatectomy remained to be the standard treatment for most patients with localised and locally advanced PCa (LAPCa) (4,5). However, one third of PCa patients might suffer biochemical relapse with a rise in serum PSA after radical prostatectomy without salvage treatment (6). Thus, combination therapy with androgen deprivation therapy (ADT) and/or salvage RT had been extensively investigated to improve the symptoms and prognosis in patients with PCa. Although some high-quality phase III clinical trials have performed to compare different combined therapeutic modalities of LAPCa, experts have not yet reached a consensus (7-11).
To explore the best comprehensive management model of patients with LAPCa, several head-to-head meta-analyses had been performed to clarify this point of view in the past years (12-14). However, these studies could merely contain two trial arms and their results remained inconclusive or unclear. Hence, this network meta-analysis was conducted to comprehensively evaluate the relative efficacy of different therapeutic modalities while respecting randomization (15,16). Ultimately, five different therapeutic modalities were enrolled: RT, long-term ADT (LTADT), RT + short-term ADT (RT + STADT), RT + LTADT and RT + orchiectomy; and seven different clinical outcomes were analyzed: overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), cancer-specific survival (CSS), local progression rate (LPR), distant failure/metastasis rate (MR) and biochemical failure rate (BFR). The results of our analysis could provide a hierarchy of five different regimens, and based on which, clinician could choose an optimal therapeutic paradigm.
Methods
Literature search
A systematic literature searched on PubMed, EMBASE and Web of Science was performed to identify all published potentially appropriate studies until September 1st, 2017. The search strategy consisted of seven parts (OS, DFS, PFS, CSS, LPR, distant failure/MR and BFR), using the following keywords for searching in combination with Medical Subject Headings (MeSH) terms: “locally advanced prostate cancer (or LAPCa)”, “hormone blockade”, “endocrine treatment”, “androgen deprivation therapy (or ADT)”, “radiotherapy (or RT)”, “orchiectomy”, “Radical prostatectomy or RP” and randomized controlled trials (RCTs). Besides, additional publications were identified manually, when we searched relevant reviews and the reference list of original articles. Furthermore, because of the data from previously published studies, ethical approval and informed consent were not required.
Study selection criteria
Articles had to meet the following criteria were included in this meta-analysis: (I) RCTs (prospective or retrospective); (II) the language of the article was limited to English; (III) patients were diagnosed as LAPCa; (IV) the included studies should address the survival of therapeutic modalities of LAPCa by assessing OS or CSS or PFS or DFS or LPR or MR or BFR.
In addition, studies would be excluded if they meet the following criteria: (I) the language of the article was non-English; (II) the publication type of study were reviews or letters or case reports or comments or editorials; (III) non-sufficient and unavailable data could extracted for our analyses in these articles; (IV) duplication of previous publications.
Data extraction
Two blind reviewers (ZQ Qin and Y Wang) individually extracted all available data involved in eligible references according to the study selection criteria mentioned above. Any disagreement was resolved by consensus and discussion. If consensus could not be reached, a third investigator (YX Zheng) acted as an arbitrator until a consensus was reached. The following information was recorded for each selected study: name of first author, year and journal of publication, study name and/or trial number, management model in each clinical trial arms and number of patients and primary endpoints of each study. All of the aforementioned data were comprehensively presented in Tables 1,2.
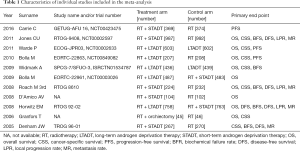
Full table
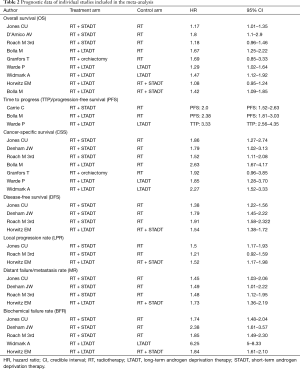
Full table
Risk of bias assessment
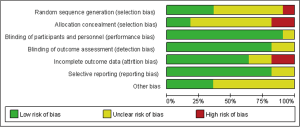
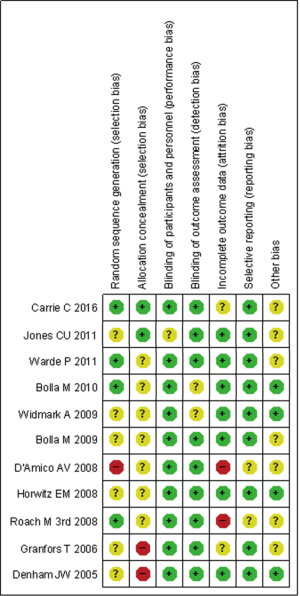
Two investigators independently evaluated the quality of each reference according to the Cochrane Handbook (17). In addition, the quality of eligible studies was evaluated the potential source of bias as follows: (I) random sequence generation; (II) allocation concealment; (III) blinding of participants and personnel; (IV) blinding of outcome assessment; (V) incomplete outcome data; (VI) selective reporting; (VII) other bias. The judgments were graded as a low, high or unclear risk of bias (http://www.cochrane-handbook.org). Ultimately, the results presented as a risk of bias summary and a risk of bias graph (Figures 1,2).
Statistical analysis
A pair-wise meta-analysis was performed to make direct comparison between two PE oral drugs, and the results were evaluated by the hazard ratio (HR) with corresponding 95% credible interval (CI). I-square test was adopted to assess the heterogeneity and I2>50% was considered as existence of significant heterogeneity. Z test was performed to determine the statistical significance and a P value of 18). In addition, all above statistical analyses in traditional meta-analysis were conducted by Stata software (version 12.0; StataCorp LP, College Station, TX, USA).
We used Der Simonian-Laird random-effects model in conventional pairwise meta-analysis (19). To incorporate direct and indirect evidence into a single comparison, we performed a random-effects network meta-analysis was conducted based on a Bayesian framework and used Markov chain Monte Carlo methods to obtain pooled estimates by using package “gemtc” version 0.8.2 of R-3.4.0 software (16,20). Next, network plots were generated to demonstrate the comparison scheme for each LAPCa therapeutic modalities. The HR with 95% CI was calculated by Markov chain Monte Carlo methods. The function mtc.run would be used to generate samples by means of the Markov chain Monte Carlo sampler. We set 10,000 simulations for each chain as the “burn-in” period, yielding 50,000 iterations to obtain the HR of model parameters, when three Markov chains run simultaneously. The model convergence was accessed by Brooks-Gelman-Rubin plots method, trace plot and density plot (Figures S1,S2) (21). Meanwhile, rank probabilities would be calculated, which indicated the hierarchy of each treatment. Based on the results of rank probabilities, clinical surgeons could make the choice which treatment would be best, second and so on (22). The matrix as well as the plot of the treatment rank probabilities would be provided by the “gemtc” package simultaneously.
Besides, the pooled HRs from network meta-analysis and traditional meta-analysis were used to estimate the consistency between direct and indirect comparisons. To access the inconsistency, the node-splitting method was applied by reporting its Bayesian P value, by means of separating the evidence concerning certain comparison into direct and indirect evidence, when a loop connecting three arms existed (23). Last but not least, the mtc.anohe command of the “gemtc” package would be utilized to evaluate the global heterogeneity on the bias of the magnitude of heterogeneity variance parameter I2.
Results
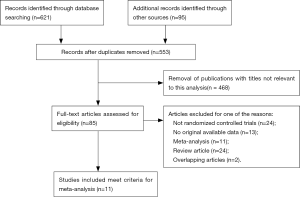
A total of 716 studies identified by previous search strategy were enrolled in the present network meta-analysis. Then, full text screen was carried out and 468 studies were excluded since they were reviews, duplicate reports and conference articles. Seventy-four articles were disregarded after titles and abstracts filtering. Finally, 11 articles including a total 8,998 patients were included in our study for further evaluation, which had been accrued between March 2002 and February 2017 (7-10,24-30). This included studies covered five different therapeutic modalities: RT, LTADT, RT + STADT, RT + LTADT, RT + orchiectomy; and seven different clinical outcomes: OS, DFS, PFS, CSS, LPR, MR and BFR. All of these enrolled studies were RCTs and the quality of evidence was evaluated by the Cochrane Handbook (Figures 1,2).The flowchart of literature search and selection procedure was shown in Figure 3. In addition, the network structure diagrams were displayed in Figure 4. Meanwhile, the thicknesses of the lines were proportional to the number of comparisons, and the diameters of the circles were proportional to the number of treatments included in the network meta-analysis.
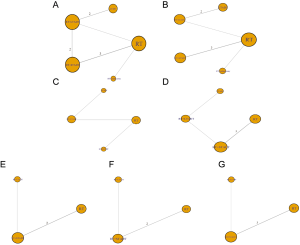
OS
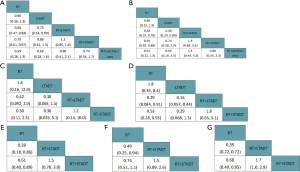
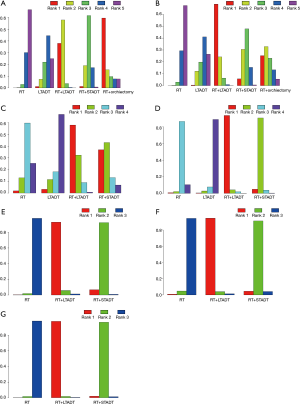
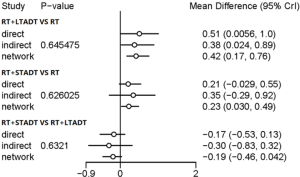
The results of OS were calculated by 9 studies including 5 therapeutic modalities (RT, LTADT, RT + STADT, RT + LTADT and RT + orchiectomy) and the network structure diagrams were displayed in Figure 4A. The efficacy of different therapeutic paradigms for HRs and corresponding 95% CIs was detailed in Figure 5. As indicated in the result, RT + LTADT showed better survival benefit, compared with RT or LTADT (HR =0.66, 95% CI: 0.47–0.84; HR =0.73, 95% CI: 0.54–0.99, separately); RT + STADT displayed a longer OS, compared with RT (HR =0.79, 95% CI: 0.61–0.97). The cumulative rank probability of five therapeutic regimens from best to worst was RT + orchiectomy > RT + LTADT > RT + STADT > LTADT > RT (Figure 6). In the above-mentioned study, we found that all Bayesian P values of node-splitting method were greater than 0.05 in terms of OS, which indicated that the direct and indirect evidence was consistent (Figure 7).
CSS
A total of seven studies including 5 therapeutic modalities (RT, LTADT, RT + STADT, RT + LTADT and RT + orchiectomy) contributed to the analysis of CSS. The network structure diagrams were presented in Figure 4B and the efficacy of five therapeutic paradigms was shown in Figure 5B. Meanwhile, RT + LTADT showed better survival benefit, compared with RT or LTADT (HR =0.38, 95% CI: 0.19–0.78; HR =0.48, 95% CI: 0.29–0.80, respectively); RT + STADT displayed a longer CSS, compared with RT (HR =0.59, 95% CI: 0.39–0.88). The cumulative rank probability from first to last was RT + LTADT > RT + orchiectomy > RT + STADT > LTADT > RT (Figure 6B). Due to the absence of a loop connecting three arms, the node-splitting method was not calculated.
DFS or BFR
The efficacy of four different therapeutic modalities (RT, LTADT, RT + LDADT and RT + STADT) was also compared in the terms of PFS and BFR, and network structure diagrams were presented in Figure 4C,D, separately. The efficacy of four therapeutic paradigms for HRs and corresponding 95% CIs was presented in Figure 5C,D. We could easily found that, in the case of BFR, RT + LTADT showed longer survival, compared with RT or LTADT (HR =0.29, 95% CI: 0.084–0.91; HR =0.16, 95% CI: 0.057–0.44, respectively); RT + STADT displayed better survival, compared with RT (HR =0.53, 95% CI: 0.28–0.93). The results of cumulative probability sorting were RT + LTADT > RT + STADT >RT > LTADT and RT + LTADT > RT + STADT > RT > LTADT in PFS and BFR, respectively (Figure 6C,D). The node-splitting method was omitted, because of the absence of a loop connecting three arms.
PFS, LPR or MR
Since the limited data, we compared only three different therapeutic modalities (RT, RT + LDADT and RT + STADT) in the terms of DFS, LPR or MR, and the networks of comparisons were shown in Figure 4E,F,G, separately. Moreover, the efficacy of different therapeutic paradigms for HRs and corresponding 95% CIs was presented in Figure 5E,F,G, respectively. Obviously, we found that the cumulative rank probability from best to worst was RT + LTADT > RT + STADT > RT, RT + LTADT > RT + STADT > RT and RT + LTADT > RT + STADT > RT in DFS, LPR and MR, respectively (Figure 6E,F,G). Owing to the absence of a loop connecting three arms, the node-splitting method could not be applied.
Node-splitting method
When a loop connecting three arms existed, the node-splitting method was implemented by reporting its Bayesian P value, by means of separating the evidence concerning certain comparison into direct and indirect evidence, to access the inconsistency. In the above-mentioned study, we found that all Bayesian P values of node-splitting method were greater than 0.05 in terms of OS, which indicated that the direct and indirect evidence was consistent (Figure 7).
Discussion
Technological and medicinal advancement has widened treatment options for LAPCa. Apart from radical prostatectomy, RT and ADT was crucial portion of comprehensive treatment strategy of the diseases. Although these therapeutic methods had supported by several randomized phase III clinical trials, the lack of comprehensive comparison of efficacy limited the clinical application of the treatments (7,24,31,32). Therefore, in our network meta-analysis, 11 relevant articles were enrolled and five different therapeutic modalities (RT, LTADT, RT + STADT, RT + LTADT or RT + orchiectomy) and seven different clinical outcomes were ultimately analyzed. Due to the absence of relevant studies on radical prostatectomy in the RCTs, radical prostatectomy was not involved in this article. Among the involved five different therapeutic modalities, our results demonstrated that RT + LTADT or RT + orchiectomy was among the best two therapeutic regimens in the prognostic aspects of the patients with LAPCa. In other words, RT + LTADT could have a comparable survival benefit as RT + orchiectomy.
To date, radical prostatectomy remains one of the standard treatments for LAPCa in men younger than 70 years. However, 30–70% of men have biochemical relapse at 5 years, depending on their initial prognosis (4). Although no standard salvage treatment has been defined, retrospective studies have suggested a potential benefit from salvage RT or ADT with a biochemical complete response seen in half of relapsing patients (10,11,24,26,29). The RTOG 85-31 study by Pilepich et al. have demonstrated for the first time that RT combined ATD could obtain a survival benefit (33). The results of this clinical trial containing 977 patients showed that androgen suppression applied as an adjuvant after definitive RT was associated with a reduction in disease progression, and a statistically significant improvement in absolute survival was observed preferentially in PCa patients with a Gleason score of 7–10 in 10 year follow-up. The study performed by Bria et al. (34) compared applied RT alone and combination of RT and ADT in the treatment of LAPCa. Their results showed that the BFR, clinical progression, local relapse, and distant metastases were all decreased in combined treatment, to a certain degree. Meanwhile, we also found that combined treatment did not increase the risk of toxicity. In addition, other studies have reported that addition of ADT to conventional-dose RT could improve OS and CSS of the patients with LAPCa (35-37).
Recently, increasing relevant published studies revealed conflicting results about the combination of RT and ADT. It was reported that ADT had no significant clinical effect on the incidence of pelvic recurrences or OS of patient with LAPCa (38-40). However, the treatment method, ADT after RT, has the benefit from the patient with LAPCa, which was reported in a trial performed by Zagars et al. (41). In this network meta-analysis, significant benefits of PFS and freedom form metastases were discovered in RT-combined-ADT treatment arm compared with RT treatment arm. But, the data about OS of LAPCa patients were similar between these two arms.
According to the National Comprehensive Cancer Network (NCCN) guidelines, the patients with intermediate-risk PCa should be treated with a combined treatment modality including 4–6 months of ADT, whereas the patients with high-risk features benefit at maximum from long-term hormonal therapy (2–3 years) (7,24,28,42). The European Association of Urology suggested a role for radical prostatectomy and pelvic lymph-node dissection or RT plus androgen deprivation as primary treatments for high-risk or locally advanced PCa (43). Although ADT combined with RT has been increasingly accepted for clinical decision-making, the biochemical mechanism was still not fully elucidated. To date, several mechanisms include pro-apoptosis, anti-angiogenesis, and increasing the sensitivity of cells to oxidative stress were proposed (44). It was reported that ADT not only had cytoreductive properties and the potential to control micrometastatic disease, but also could enhance RT sensitivity of the lesion (44-46). This might explain the combined treatment group had better survival rate.
Of note, urologists and oncologists should balance outcomes and adverse events based on a correct assessment of cancer stage and risk to make a decision of LTADT or STADT management strategy. In the clinical trial RTOG 86-10, the patients were randomly grouped with bulky T2–T4 tumors to radiation with or without goserelin and flutamide (47). Significant benefits of OS, disease-specific mortality, distant metastasis and BFS were seen in combined treatment (8). In addition, the patients with a Gleason score of 6 or less benefited most from STADT. However, the result was conflicted with RTOG 85-31, where LTADT was delivered (48). In RTOG 94-08, RT alone or RT combined STADT were performed in 1979 patients with stage T1b to T2b PCa and PSA less or equal than 20 ng/mL (29). In this network meta-analysis, the 10-year OS was better in combined treatment (62% vs. 57%, P=0.03) and the benefit was mostly seen with intermediate-risk LAPCa patients rather than low-risk LAPCa patients. In addition, the combination of LTADT and RT was shown to significantly improve OS, compared with STADT in patients with high-risk PCa (7,27). In terms of dose-escalated RT, the study from Liauw et al. enrolled a total of 238 patients with intermediate-risk (PSA: 10–20 ng/mL, Gleason =7, or stage T2b–c) adenocarcinoma of the prostate by treating with external beam RT between 1989 and 2006, and 112 LAPCa patients (47%) received neoadjuvant and concurrent ADT (3). Moreover, the results demonstrated that intermediate-risk PCa patients with percentage of positive cores > or =50% had the highest risk for biochemical failure after dose-escalated RT, and might be most likely to derive a benefit from ADT (49).
Although the usage of ADT combined with RT was undoubtedly associated with significant clinical benefits, the occurrence of adverse events required the attention of urologist and oncologist. Complications such as vasomotor symptoms, erectile dysfunction, and impairment of cognitive function could significantly reduce the quality of life of LTADT patients (50,51). In addition, the use of LTADT was associated to an increased age-related loss of bone mineral density, which could lead to pathological fracture (52). Besides, LTADT combined RT could also increase the risk of genitourinary and gastrointestinal morbidity (53). Ultimately, the aggravated financial burden of LAPCa patients limited the performance of LTADT. It should be noted that orchiectomy had a good androgen blocking effect, whereas some patients had poor psychological receptivity. Hence, surgeons should carefully discuss with LAPCa patients to clarify the physical and psychological consequences before operation.
To a certain extent, several limitations should be paid attention to, before fully understanding this article. Firstly, the mechanism of combination therapy of RT and ADT applied in LAPCa patients had not yet fully elucidated. Secondly, the number of studies included in this study was limited. Hence, more high-quality researches need to further focus on the influence of different therapeutic methods in the future. Thirdly, among those enrolled studies, the definition of STADT and the drugs to block androgen were not consistent. Meanwhile, inclusion criteria for data of each patient in previous articles were different a lot. Fourthly, limitations were found in the included references, such as limited sample sizes, some biases and short follow-up time. Thus, further exploration in these efficacy characteristics of different therapeutic modalities for LAPCa might be conducted in subsequent years. Last but not least, Due to the absence of relevant studies on radical prostatectomy in the RCTs, radical prostatectomy was not involved in this article, though it had gained more and more attention in recent years. Accordingly, it was required that further studies could be performed to elucidate the differences in the effectiveness of different therapeutic modalities for LAPCa if individual data were available.
Conclusions
In summary, the results of the current network meta-analysis indicated that RT + LTADT or RT + orchiectomy was among the best two therapeutic regimens in the prognostic aspects of the patients with LAPCa. Furthermore, in consideration of reducing invasive treatment of eligible patients, RT + LTADT could yield better survival benefit of LAPCa patients, compared with others. Additional high-quality and multicentre large-scale RCTs are needed to further to confirm these new options in subsequent articles.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81702520).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-8. [Crossref] [PubMed]
- Litwin MS, Tan HJ. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017;317:2532-42. [Crossref] [PubMed]
- Mandel P, Tilki D, Graefen M. Radical prostatectomy in locally advanced prostate cancer. Urologe A 2017;56:1394-401. [Crossref] [PubMed]
- Dell'Oglio P, Abou-Haidar H, Leyh-Bannurah SR, et al. Assessment of the Rate of Adherence to International Guidelines for Androgen Deprivation Therapy with External-beam Radiation Therapy: A Population-based Study. Eur Urol 2016;70:429-35. [Crossref] [PubMed]
- Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281:1591-7. [Crossref] [PubMed]
- Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 2008;26:2497-504. [Crossref] [PubMed]
- Roach M, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol 2008;26:585-91. [Crossref] [PubMed]
- Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009;373:301-8. [Crossref] [PubMed]
- Warde P, Mason M, Ding K, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet 2011;378:2104-11. [Crossref] [PubMed]
- Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol 2016;17:747-56. [Crossref] [PubMed]
- Fahmy O, Khairul-Asri MG, Hadi S, et al. The Role of Radical Prostatectomy and Radiotherapy in Treatment of Locally Advanced Prostate Cancer: A Systematic Review and Meta-Analysis. Urol Int 2017;99:249-56. [Crossref] [PubMed]
- Dong Z, Wang H, Xu M, et al. Intermittent hormone therapy versus continuous hormone therapy for locally advanced prostate cancer: a meta-analysis. Aging Male 2015;18:233-7. [Crossref] [PubMed]
- Schmidt-Hansen M, Hoskin P, Kirkbride P, et al. Hormone and radiotherapy versus hormone or radiotherapy alone for non-metastatic prostate cancer: a systematic review with meta-analyses. Clin Oncol (R Coll Radiol) 2014;26:e21-46. [Crossref] [PubMed]
- Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med 2002;21:2313-24. [Crossref] [PubMed]
- Salanti G, Higgins JP, Ades AE, et al. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279-301. [Crossref] [PubMed]
- Higgins J, Green SE. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. editors. Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie 2011;2011:S38.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. [Crossref] [PubMed]
- Wu HY, Huang JW, Lin HJ, et al. Comparative effectiveness of renin-angiotensin system blockers and other antihypertensive drugs in patients with diabetes: systematic review and bayesian network meta-analysis. BMJ 2013;347:f6008. [Crossref] [PubMed]
- Gelman A, Rubin DB. Inference from Iterative Simulation Using Multiple Sequences. Stat Sci 1992;7:457-72. [Crossref]
- Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44. [Crossref] [PubMed]
- Denham JW, Steigler A, Lamb DS, et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol 2005;6:841-50. [Crossref] [PubMed]
- Roach M, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol 2008;26:585-91. [Crossref] [PubMed]
- D'Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA 2008;299:289-95. [PubMed]
- Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 2009;360:2516-27. [Crossref] [PubMed]
- Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 2010;11:1066-73. [Crossref] [PubMed]
- Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 2011;365:107-18. [Crossref] [PubMed]
- Shrayyef MZ, DePapp Z, Cave WT, et al. Hypercalcemia in two patients with sarcoidosis and Mycobacterium avium intracellulare not mediated by elevated vitamin D metabolites. Am J Med Sci 2011;342:336-40. [Crossref] [PubMed]
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002;360:103-6. [Crossref] [PubMed]
- Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys 2001;50:1243-52. [Crossref] [PubMed]
- Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys 2005;61:1285-90. [Crossref] [PubMed]
- Bria E, Cuppone F, Giannarelli D, et al. Does hormone treatment added to radiotherapy improve outcome in locally advanced prostate cancer?: meta-analysis of randomized trials. Cancer 2009;115:3446-56. [Crossref] [PubMed]
- Shelley MD, Kumar S, Wilt T, et al. A systematic review and meta-analysis of randomised trials of neo-adjuvant hormone therapy for localised and locally advanced prostate carcinoma. Cancer Treat Rev 2009;35:9-17. [Crossref] [PubMed]
- Verhagen PC, Schroder FH, Collette L, et al. Does local treatment of the prostate in advanced and/or lymph node metastatic disease improve efficacy of androgen-deprivation therapy? A systematic review. Eur Urol 2010;58:261-9. [Crossref] [PubMed]
- Sasse AD, Sasse E, Carvalho AM, et al. Androgenic suppression combined with radiotherapy for the treatment of prostate adenocarcinoma: a systematic review. BMC Cancer 2012;12:54. [Crossref] [PubMed]
- Harisiadis L, Veenema RJ, Senyszyn JJ, et al. Carcinoma of the prostate: treatment with external radiotherapy. Cancer 1978;41:2131-42. [Crossref] [PubMed]
- Rosen EM, Cassady JR, Connolly J, et al. Radiotherapy for localized prostate carcinoma. Int J Radiat Oncol Biol Phys 1984;10:2201-10. [Crossref] [PubMed]
- Perez AA, Pilepich MV, Zivnuska F. Tumor control in definitive irradiation of localized carcinoma of the prostate. Int J Radiat Oncol Biol Phys 1986;12:523-31. [Crossref] [PubMed]
- Zagars GK, Johnson DE, von Eschenbach AC, et al. Adjuvant estrogen following radiation therapy for stage C adenocarcinoma of the prostate: long-term results of a prospective randomized study. Int J Radiat Oncol Biol Phys 1988;14:1085-91. [Crossref] [PubMed]
- Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol 2003;21:3972-8. [Crossref] [PubMed]
- Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol 2008;53:68-80. [Crossref] [PubMed]
- Lu JP, Monardo L, Bryskin I, et al. Androgens induce oxidative stress and radiation resistance in prostate cancer cells though NADPH oxidase. Prostate Cancer Prostatic Dis 2010;13:39-46. [Crossref] [PubMed]
- Zietman AL, Nakfoor BM, Prince EA, et al. The effect of androgen deprivation and radiation therapy on an androgen-sensitive murine tumor: an in vitro and in vivo study. Cancer J Sci Am 1997;3:31-6. [PubMed]
- Zietman AL, Prince EA, Nakfoor BM, et al. Androgen deprivation and radiation therapy: sequencing studies using the Shionogi in vivo tumor system. Int J Radiat Oncol Biol Phys 1997;38:1067-70. [Crossref] [PubMed]
- Pilepich MV, Krall JM, Al-Sarraf M, et al. Androgen deprivation with radiation therapy compared with radiation therapy alone for locally advanced prostatic carcinoma: a randomized comparative trial of the Radiation Therapy Oncology Group. Urology 1995;45:616-23. [Crossref] [PubMed]
- Souhami L, Bae K, Pilepich M, et al. Impact of the duration of adjuvant hormonal therapy in patients with locally advanced prostate cancer treated with radiotherapy: a secondary analysis of RTOG 85-31. J Clin Oncol 2009;27:2137-43. [Crossref] [PubMed]
- Liauw SL, Fricano J, Correa D, et al. Dose-escalated radiation therapy for intermediate-risk prostate cancer: patient selection for androgen deprivation therapy using percentage of positive cores. Cancer 2009;115:1784-90. [Crossref] [PubMed]
- Collins L, Basaria S. Adverse effects of androgen deprivation therapy in men with prostate cancer: a focus on metabolic and cardiovascular complications. Asian J Androl 2012;14:222-5. [Crossref] [PubMed]
- Brundage M, Sydes MR, Parulekar WR, et al. Impact of Radiotherapy When Added to Androgen-Deprivation Therapy for Locally Advanced Prostate Cancer: Long-Term Quality-of-Life Outcomes From the NCIC CTG PR3/MRC PR07 Randomized Trial. J Clin Oncol 2015;33:2151-7. [Crossref] [PubMed]
- Bruder JM, Ma JZ, Basler JW, et al. Prevalence of osteopenia and osteoporosis by central and peripheral bone mineral density in men with prostate cancer during androgen-deprivation therapy. Urology 2006;67:152-5. [Crossref] [PubMed]
- Wilcox SW, Aherne NJ, Benjamin LC, et al. Long-term outcomes from dose-escalated image-guided intensity-modulated radiotherapy with androgen deprivation: encouraging results for intermediate- and high-risk prostate cancer. Onco Targets Ther 2014;7:1519-23. [Crossref] [PubMed]