Anaphylactic cardiovascular collapse and Kounis syndrome: systemic vasodilation or coronary vasoconstriction?
Introduction
Anaphylaxis (from Hellenic anaphylaxis = no phylaxis, meaning without protection, instead of prophylaxis meaning protection), is referred to the event cascade that may follow exposure to a particular antigen and causing an acute multi-organ response, with cardiac, coronary/systemic arterial, dermatological (although skin features being absent in up to 20% of people), respiratory, renal, gastrointestinal and neurological involvement (1). Consequently, anaphylaxis is regarded today as a severe, life-threatening generalized or systemic hypersensitivity reaction.
Anaphylactic shock represents an emergent medical condition, thus rendering rapid diagnosis and prompt treatment life-saving (2). It is characterized by profound venous tone reduction and fluid extravasation, further leading to reduced venous return and cardiac output suppression (3). Several reports have revealed that the heart, and coronary arteries, could represent the primary targets of anaphylaxis (4). Coronary vasoconstriction and myocardial tissue hypoperfusion lead to cardiac output reduction and peripheral tissues hypoperfusion, thus resulting in further cellular injury and tissue dysfunction. Myocardial dysfunction might constitute the anatomical basis of the irreversible cardiac failure occasionally associated with systemic anaphylaxis (5).
In this review, the key immunological, clinical, laboratory and experimental features of cardiac anaphylaxis and anaphylactic cardiovascular collapse are described in an effort to delineate the controversial role between systemic vasodilation and coronary vasoconstriction as the basis of systemic and cardiac anaphylaxis.
Historical aspects
The first human anaphylactic death is supposed to be that of Pharaoh Menes in the year 2600 BC, attributed to the sting of a wasp or hornet, while he was travelling to the British Isles (6). This event is based on hieroglyphs of two only partially preserved but almost identical ebony plates found at one of the many putative Menes burial sites (7).
In the modern world, the correlation between cardiovascular disorders and anaphylactic reactions was demonstrated in the previous century (8) in animal experimental models (9), which revealed electrocardiographic and other direct alterations of cardiac activity. The term cardiac shock was introduced, at the same period, as a physiological entity referring to feeble pulse and low blood pressure as vascular shock manifestations. Combination of the two was referred as cardiovascular shock. This term was applied to the anaphylactic reactions documented in dogs and rabbits, but not to that of guinea pigs, as the latest were usually dying from asphyxia during experiment (10). Cardiovascular symptoms and signs, accompanying allergic, hypersensitivity, anaphylactic or anaphylactoid reactions, firstly appeared in the English, German and Austrian medical literature >7 decades ago. These reactions were attributed to serum sickness and tetanus antitoxin and were characterized as carditis with morphologic cardiac lesions (11), serum reactions inducing acute carditis (12), or lesions with basic characteristics of rheumatic carditis (13).
In 1950, Czickeli (14), was the first who attempted to correlate allergic reactions with physiological mechanisms of angina pectoris and acute myocardial infarction.
In the same year, Pfister and Plice (15) described the first true acute myocardial infarction associated with urticaria in a 49-year-old man treated with 300,000 units/day penicillin in oil. Treatment with dicumarol, papaverine, morphine and diphenhydramine hydrochloride demonstrated favorable results in management of this patient.
In 1965, Zosin et al. (16) published the first report of allergic myocardial infarction regarding a 48-year-old atopic male patient admitted with urticarial rash, retrosternal tightness, premonition of imminent death and shock following egg, milk and strawberries consumption as well as sulfonamide and pyramidon administration. The patient was treated with antihistamines and was discharged after 30 days of hospitalization in a good general condition. In 1991, the allergic angina syndrome was defined (17) as “the coincidental occurrence of chest pain and allergic reactions, accompanied by clinical and laboratory findings of classical angina pectoris caused by inflammatory mediators released during the allergic insult”. The authors suggested that the mechanism of chest pain development in patients suffering an allergic reaction could be explained by the underlying coronary arterial spasm induced by the release of mast cell mediators including histamine. Today, this constitutes the type I variant of Kounis syndrome (18). This variant of angina could progress to acute myocardial infarction called “allergic myocardial infarction” and representing the type II variant of Kounis syndrome (19). Kovanen et al. (20) demonstrated a much higher (200:1) degree of mast cell degranulation at the sites of plaque erosion or rupture compared to adjacent areas or even more distant segments of unaffected arteries, in patients died of acute myocardial infarction of non-allergic etiology. This report rendered clear the existence of a common pathway between allergic and non-allergic coronary events that could have profound therapeutic and clinical implications. In the meantime, Constantinides (21) raised the possibility that even ordinary allergic reactions could promote plaque disruption, whereas Brawnwald (22) categorized allergic angina as a subgroup of dynamic coronary occlusion lesions, where allergic reactions with mediators such as histamine or leukotrienes acting on coronary vascular smooth muscle can induce vasospastic angina.
Finally, the first comprehensive report, concerning the unique association of anaphylaxis and the heart was published in 2006 (23) characterizing these events as “magnificent nature’s own experiment”. During this year a potential relationship between drug-eluting coronary stent thrombosis and hypersensitivity to coronary stent components as a manifestation of Kounis syndrome was emphasized (24).
Anaphylactic shock: pathophysiologic considerations
The recent International Consensus on Anaphylaxis (25), defined anaphylaxis as “a serious, generalized or systemic, allergic or hypersensitivity reaction that can be life-threatening or fatal.” Indeed, anaphylaxis represents an important potential cause of life threatening events. According to recent reports, 2–3% of the general population will experience anaphylaxis during their lifetime (26,27).
Allergic or hypersensitivity reactions and anaphylaxis constitute immune mediated conditions, sharing the same underlying pathophysiologic mechanisms. Previous exposure to an antigen or a substance of similar structure might be the cause of a subsequent anaphylactic reaction. The presence of inflammatory mediators released by mast cells and basophils, in case of an allergen interaction with membrane-bound immunoglobulin E (IgE), constitutes the basis of acute allergic reactions, thus including anaphylaxis (28). Mast cells, mainly, and basophils, occasionally, are triggered to release their inflammatory mediators, when a specific number of high affinity IgE receptors FceRI (fragment crystallizable region) is aggregated. Such a concentration in density of FceRI receptors can be achieved by the bridging of IgE antibodies to an allergen disposing at least two epitopes corresponding to the IgE antibodies (29). The critical number of bridged IgE molecules has been estimated to be 2,000, thus creating 1,000 bridges, out of a maximal number of 500,000–1,000,000 IgE molecules on cell surface (30).
Anaphylactic reactions associated with cardiovascular alterations are frequent and transient, but in some cases they may result in extensive and life-threatening myocardial damage (31). Fact that can be attributed to the large number of mast cells that are located in the tunica media and adventitia of large coronary vessels and around small intramural coronary arteries (32). During anaphylaxis, coronary hypoperfusion caused by systemic vasodilation, plasma leakage, loss of volume due to increased vascular permeability and reduced venous return can contribute to cardiac output suppression, leading further (33) to myocardial damage and ventricular dysfunction.
Various mechanisms have been incriminated in order to explain the cardiovascular collapse during anaphylaxis (34), including excessive activation of vasodilators and increased nitric oxide (NO) synthesis. NO activates soluble guanylate cyclase and increases cyclic guanosine monophosphate. Furthermore, elevated prostacyclin synthesis activates soluble adenylate cyclase and produces cyclic adenosine monophosphate. Finally, shock induced acidosis and NO release activate vascular potassium channels, thus further causing severe and persistent vasodilation resistant to catecholamine administration (35).
During anaphylactic shock, circulating blood volume might be reduced by 35% within 10 min due to intravascular fluid extravasation (36). Furthermore, severe vasodilation resistant to epinephrine that might respond only to potent vasoconstrictors has been also reported (37). This shift of fluid volume is counteracted by compensatory vasodepressor mechanisms involving release of epinephrine and norepinephrine (38) together with activation of angiotensin system (39).
Excessive catecholamine production might have various effects. Some patients during anaphylactic episodes may present decreased systemic vascular resistance, while others experience maximum peripheral vasoconstriction due to increased vascular resistance (40). Epinephrine injections sometimes fail to treat severe acute allergy and this is attributed to the effect of various internal compensatory mechanisms. Furthermore, the endogenous catecholamine release enhanced further by exogenous adrenaline administration can have an adverse impact on myocardium, thus triggering ischemic chest pain and electrocardiographic changes even in the absence of coronary artery disease (41,42). Indeed, platelets of angina pectoris patients are more sensitive to increased endogenous serum epinephrine levels, provoking platelet activation and aggregation as well as thrombosis induction (43).
Experiments and clinical reports, support that the human heart, in general, and the coronary arteries, in particular, could be the site and the primary target of anaphylaxis, raising questions regarding the etiology of cardiovascular collapse during anaphylaxis. Could coronary hypoperfusion be the result of systemic vasodilation induced by plasma leakage, volume loss due to increased vascular permeability, and reduced venous return? or could coronary vasoconstriction and thrombosis induced by anaphylactic mediator release (histamine, chymase, tryptase, cathepsin D, leukotrienes, thromboxane, PAF) lead to myocardial damage with increase of cardiac biomarkers (troponin), heart failure and reduced cardiac output?
Indeed, histamine can induce coronary vasoconstriction via the H1 receptors (17), chymase converts angiotensin I to angiotensin II constituting a major vasoconstriction substance, as cathepsin-D does (44), tryptase activates the zymogen forms of metalloproteinases such as interstitial collagenase, gelatinase, and stromelysin that can promote plaque disruption or rupture as well as induction of pericellular matrix degradation (basophils have about 500-fold lower levels of tryptase than mast cells) (45). While leukotrienes act as powerful vasoconstrictors (46), thromboxane promotes platelet aggregation and vasoconstriction (47) and finally PAF can further induce arrhythmias, ST segment changes and vasoconstriction (48) (Figure 1).
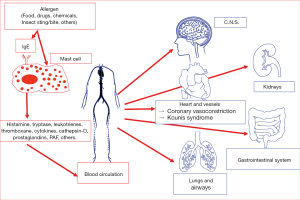
The heart as primary target of anaphylaxis
Experimental evidence
Several experimental studies revealed that the primary targets of anaphylaxis could be the heart and especially the coronary arteries.
- In the first ever published experimental study (49), severe cardiovascular dysfunction was reported to occur primarily during systemic anaphylaxis. This study clearly delineated the cardiac involvement in systemic anaphylactic reactions, demonstrating the relevance of immunologically-induced cardiovascular collapse. Indeed, the primary cardiac reactions induced by the intracardiac histamine release were followed by secondary cardiovascular reactions caused by systemic release of anaphylactic mediators.
- In experimental anaphylaxis with guinea pig hearts, the following events occurred post intra-aortic injection of the antigen: (i) quick and prolonged decrease of coronary blood flow; (ii) abrupt increase of heart rate, peaked within 2 minutes; (iii) transient increase in ventricular contractility, followed by a prolonged decrease, reaching its nadir 6–8 minutes after antigen challenge; (iv) Arrhythmias such as idioventricular rhythm and conduction defects, ranging from partial to complete atrioventricular block occurred 45 seconds after antigen administration and lasted for about 9 minutes (50).
- The hypothesis that cardiac tissue might be a target for antigen/antibody reactions was investigated in guinea pigs that were passively sensitized by intravenous anti-ovalbumin rabbit serum. Twenty-four hours later their hearts were excised and isolated according to a working heart preparation technique. Anaphylaxis was induced by a bolus injection of ovalbumin. Coronary flow, aortic flow, left ventricular developed pressure and its first derivative (LVdp/dtmax) were profoundly decreased after induction of anaphylaxis. A significant increase in heart rate and left ventricular end diastolic pressure (LVEDP) were also observed. All these alterations occurred within the first 5 minutes, whereas all variables returned to approximately the pre-challenge values. When a specific PAF receptor antagonist was administered prior to the anaphylactic challenge, heart rate and LVEDP increase, whilst coronary and aortic flow, left ventricular developed pressure and LVdp/dtmax reduction were all inhibited in a dose-dependent manner (51).
- In ovalbumin-sensitized guinea pig experimental anaphylaxis model within 3 minutes post ovalbumin administration several cardiac features were changed as follows (52):
- decrease of cardiac output by 90%;
- significant increase of LVEDP by 35%, indicating pump failure;
- significant increase of arterial blood pressure by 35% that started declining steadily after 4 minutes;
- acute myocardial ischemia confirmed by concurrent electrocardiographic changes. The authors concluded that “the idea that the registered anaphylactic damage might be due to peripheral vasodilatation can be definitely excluded”. In addition, the rapid LVEDP increase suggests that decreased venous return and volume loss due to vascular permeability increase are unlikely to be the primary causes of the documented cardiac output and blood pressure depression.
- Experiments in rats (53) have shown that the vascular resistance responses to anaphylactic shock are characterized by considerable increase in portal venous resistance, with only initial transient decrease in hepatic artery and splanchnic vascular resistances. An absence of a significant increase in splanchnic vascular resistances was evident only during the early stage of the experiment. Fact that opposes to the general belief that anaphylactic shock is the result of diminished vascular resistance, leading to vasodilatation and fluid extravasation. The elevated portal venous resistance could be attributed to anaphylaxis-released vasoactive mediators, which can constrict portal veins in isolated perfused rat livers (54).
- In sensitized rats by subcutaneous administration of egg albumin the mean arterial pressure, carotid blood flow, cardiac output, cerebral cortical blood flow (estimated by laser Doppler technique) and cerebral tissue oxygen pressure were recorded over the 15 minutes following anaphylactic shock induction. All these parameters were decreased, but the authors of this article concluded that the severe cerebral blood flow impairment could not be explained by the level of arterial hypotension during anaphylactic shock (55). In their experimental rat model of anaphylaxis, the tissue oxygen partial pressure decreased very rapidly, as early as 1 minute following anaphylaxis onset, fact that was attributed to early and direct action of anaphylactic mediators on cerebral vessels. Indeed, reduced cerebral blood flow, leading to post-ischemic hypoperfusion could be the result of mast cell mediator release such as histamine, chymase, leukotrienes and platelet-activating factor, which could further induce cerebral artery spasm during anaphylaxis. Therefore, cerebral ischemia and brain injury following anaphylactic shock could be due to direct action of anaphylactic mediators on cerebral arterial system and not solely due to arterial hypotension (56).
- Cardiac anaphylaxis was induced by intracoronary injections of ovalbumin in isolated hearts of ovalbumin-sensitized Wistar rats. In response to ovalbumin administration, the sensitized rat hearts of anaphylaxis group demonstrated a decrease in coronary blood flow, and in the maximum rate of systolic left ventricular pressure (dP/dtmax) and an increment in coronary vascular resistance, as evidence of coronary spasm. The authors concluded that these results may suggest that left ventricular dysfunction during anaphylaxis can be attributed mainly to coronary vasoconstriction and the inducible myocardial ischemia (57,58).
- In other experimental models, anaphylaxis was induced by ovalbumin antigen injection into open-chest of artificially ventilated sensitized mice. Aortic blood flow, mean arterial pressure, pulmonary arterial pressure, left atrial pressure and central venous pressure were continuously measured (59). In the sensitized mice, the aortic blood flow and mean arterial pressure underwent an initial transient increase, followed by progressive decrease post the antigen injection. No total peripheral resistance reduction was observed, while pulmonary artery pressure showed initially a transient increase up to 18.5±0.5 mmHg along with pulmonary vascular resistance increment. The aortic blood flow and the mean arterial pressure decrease induced by the antigen were attenuated by pretreatment with either a platelet-activating factor receptor antagonist, or by histamine H1 receptor antagonist, -diphenhydramine-, and further were abolished by their combination. Diphenhydramine enhanced the initial pulmonary artery pressure and pulmonary artery resistance, without affecting the corresponding mean arterial pressure drop. Furthermore, administration of either leukotriene C4 or serotonin antagonists, alone or in combination with the platelet-activating factor receptor antagonist, revealed no significant impact. The authors again concluded that mice anaphylactic hypotension could be only attributable to cardiac output reduction via PAF and histamine actions and not to vasodilation. Conversely, the slight increase in pulmonary artery pressure was not involved in mice anaphylactic hypotension.
- A recent experimental study (60), aiming to elucidate the role of nitrtic oxide as a potential therapeutic target in cardiac anaphylaxis, was undertaken in albumin-sensitized mice and wild-type mice.
The hearts of male mice were excised and were perfused retrogradely with Krebs-Henseleit solution, at a constant perfusion pressure, according to the Langendorff technique. Cardiac anaphylaxis was elicited by ovalbumin solution injection into the aortic cannula. Coronary flow was measured and oxidative stress markers such as index of lipid peroxidation as thiobarbituric acid-reactive substances, NO, superoxide anion radical and hydrogen peroxide were assessed spectrophotometrically in the coronary venous effluent. Post ovalbumin challenge, coronary flow was significantly decreased in the wild mice group, while NO and hydrogen peroxide were significantly increased in the mice group.
The authors concluded that coronary vasoconstriction during cardiac anaphylaxis does not necessarily depend upon inducible NO synthase activity and that NO may not be the only influential mediator of anaphylactic reaction in isolated mice heart
Clinical evidence
The following clinical studies demonstrate that cardiac biomarkers indicative of myocardial injury are increased in the various types of anaphylaxis. Furthermore, there are also clinical reports of patients with anaphylactic cardiac collapse, manifesting as Kounis syndrome and Takotsubo cardiomyopathy accompanied with increased serum cardiac troponin, who did not respond to intravenous fluid administration and inotropic support and required coronary syndrome treatment protocol.
- In a recent clinical study (61) of 31 patients admitted to the emergency department suffering from anaphylaxis, angioedema, urticaria and urticaria angioedema, it was demonstrated for the first time a significantly increased cardiac troponin I concentration compared to 125 healthy controls. In the subgroup of anaphylaxis, the cardiac troponin I levels were higher than those of patients with milder allergic reactions. The systematic cardiac troponin measurement in patients with acute allergic reactions in order to detect and treat potential myocardial injury was necessary according to the authors. Indeed, these findings might have profound clinical, therapeutic and pathophysiologic implications as far as anaphylaxis, myocardial injury and Kounis anaphylaxis-associated acute coronary syndrome (62) are concerned.
- In another recent study (63) concerning 300 patients with anaphylactic reactions, admitted to the emergency department and assessed by electrocardiography, echocardiography and cardiac troponin measurements, myocardial injury was observed in 22 patients (7.3%). Various cardiomyopathies, including Kounis syndrome and Takotsubo cardiomyopathy have been also observed in patients with myocardial injury. Insect bites, drugs and food were the most frequent causes of anaphylaxis. Urticaria, dyspnea, chest pain and swollen oropharynx were the common symptoms and signs at initial presentation. No mortality was observed in the myocardial injury group, but there were 9 patients with documented cardiac arrest. The previous cardiac status and the arrest cause were not determined, as autopsy was not performed. However, one patient died due to multiple organ failure. The authors concluded that further prospective studies are required to investigate the prognosis of cardiac injury in patients with anaphylaxis.
- Another patient with hymenoptera sting-induced Kounis syndrome was complicated with anaphylactic shock. Immediate coronary angiography revealed acute coronary thrombosis and the patient underwent coronary angioplasty and supported hemodynamically with intra-aortic balloon pump following an acute coronary syndrome therapeutic protocol. The patient was eventually stabilized with optimal cardiac, antiplatelet and anti-arrhythmic medications, without anaphylaxis treatment administration and was discharged with an outpatient clinic follow-up appointment (64).
- In another report regarding a patient stung by multiple wasps and developing type I variant of Kounis syndrome with anaphylactic shock and myocardial ischemia, the administration of antihistamines, hydrocortisone normal saline and adrenaline did not have any immediate effect, and the patient recovered in a later stage with vasodepressors and myocardial infarction protocol treatment (65).
- An atopic female nurse allergic to milk protein, with eczema and bronchial asthma, developed anaphylactic reaction with hemodynamic collapse and myocardial stunning leading to reduced cardiac output (66). The hemodynamic status was unresponsive to intravenous administration of fluids such as Ringer acetate and anti-allergic treatment with adrenaline and corticosteroids. The condition was complicated by pulmonary oedema and decompensated heart failure. In this patient, application of myocardial infarction protocol treatment proved to be lifesaving.
- Metaraminol and epinephrine not only resulted to hypotension deterioration, in a patient with peri-operative anaphylactic shock due to gelofusine infusion and increased troponin, but suppressed the cardiac output within 3 minutes (67). The patient recovered gradually following administration of intravenous antihistamines and corticosteroids and ongoing inotropic support.
- In a patient with stent thrombosis post snake bite anaphylaxis associated with acute myocardial infarction, administration of inotropes and fluid expansion were not beneficial and the patient underwent myocardial infarction protocol treatment with thrombolysis with good outcome (68).
The above studies and reports are in accordance with previous laboratory and clinical evidence supporting that inflammatory mediators released locally and in the systemic circulation during allergic and anaphylactic reactions could cause coronary vasoconstriction, then leading to reduced coronary blood flow and myocardial damage. This coronary hypo-perfusion can further induce anaphylactic cardiac collapse and cerebral hypo-perfusion. Indeed, the following biogenic amines such as histamine, enzymes such as the neutral proteases chymase, tryptase, cathepsin-D, peptides, proteoglycans, chemokines, cytokines, growth factors and arachidonic acid products such as leukotrienes, thromboxane, prostacyclin, PAF and tumor necrosis factor-α are released during mast cell activation have been found to have important cardiovascular effects (69) (Table 1). All these findings denote that the heart and especially the coronary arteries may constitute the primary targets of allergic inflammatory mediators that could further induce coronary injury with increased cardiac troponin levels.

Full table
Since the coronary arteries are potential anaphylaxis targets, it is not surprising that an acute coronary syndrome may represent a part of the anaphylactic reaction or one of its predominant clinical features (70). The Kounis anaphylaxis-associated acute coronary syndrome is a typical example.
Kounis syndrome: a primary cause for the anaphylactic cardiovascular collapse
This syndrome combines acute coronary events including coronary spasm, acute myocardial infarction and stent thrombosis, with mast cell and platelet activation conditions (71). Interrelated and interacting inflammatory cells, such as mast cells, macrophages and T-lymphocytes, might be involved in an anaphylaxis cascade, then leading to Kounis syndrome. In this activation cascade, a subset of platelets is also involved via FcγRI, FcγRII, FcεRI, and FcεRII receptors situated on platelet surface (66). Kounis syndrome is caused by inflammatory mediators such as histamine, neutral proteases, platelet-activating factor, arachidonic acid products along with a variety of cytokines and chemokines released during the anaphylactic activation process (72). Despite that mast cells represent a numeric minority in this inflammatory cascade, they could decisively influence the inflammatory process. A vicious inflammatory cycle seems to exist in which all these inflammatory cells participate, activate each other via multidirectional signals. Most of these mediators may exert important cardiovascular actions. These pre-formed and newly synthesized inflammatory mediators are released locally and enter the systemic circulation, causing either coronary artery spasm which can progress to acute myocardial damage, or immediate coronary thrombosis that constitutes the main clinical manifestations of Kounis syndrome.
Incidence
Kounis syndrome does not seem to be a rare disease. However, it is infrequently recognized and underdiagnosed in clinical practice, and further reported in the medical literature. There is a paucity of large prospective trials aimed at determining the exact incidence and prevalence of this condition.
A recent prospective study (73) reported that out of 138,911 patients admitted to the emergency department during 1 year, 793 presented with allergy signs. Out of these, 769 were admitted with urticaria and 24 with angioneurotic edema. Therefore, the incidence of allergy admissions during 1 year was 5.7 per 1,000 patients. The incidence of Kounis syndrome documented in the emergency department among all admissions was 19.4 per 100,000 (27/138,911), and 3.4% among allergy patients (27/793). Of 51 cases of Kounis syndrome reported to the International Pharmacovigilance Agency (VigiBase™) during the period 2010–2014, almost half (22 reports) occurred in 2014. Most cases occurred in the USA, and non-steroidal anti-inflammatory medications were the most frequent trigger drugs (74). In a recent review article on epidemiology, diagnostic findings, management and complications of allergic acute coronary syndrome, the variant of Kounis syndrome referring to patients with normal or nearly normal coronary arteries represented the most common type (72.6%) of this syndrome. This variant can progress to coronary thrombosis leading to major adverse cardiovascular events (MACE) including cardiogenic anaphylactic shock (2.3%), cardiac arrest (6.3%) and even death (2.9%) due to ventricular fibrillation, anterior or inferior ST segment elevation myocardial infarction (STEMI) (75). The annual incidence of Kounis syndrome in patients with anaphylaxis was 2% (2 of 100) from 2012 to 2017 based on emergency department records of one Japanese hospital (76). One of Kounis syndrome patients survived, but the other died from cardiac arrest. In another Japanese hospital (77), the annual incidence of Kounis syndrome in anaphylaxis patients was 2.2% (3 of 138) at the emergency department from 2013 to 2017. All 3 patients survived, except of one that complicated with anoxic encephalopathy induced by cardiac arrest. These 2 cases, in both hospitals, who died from cardiac arrest had been caused by drug administration. The conclusion was that Kounis syndrome should be excluded when physicians treat patients with anaphylaxis (78).
Recently, the food and drug administration (FDA) Adverse Event Reporting System (2004–June 2016), a consolidated pharmacovigilance source of monitoring rare but serious adverse events such as Kounis syndrome through disproportionality analysis (79) showed that: out of 499 spontaneous reports of drug-induced Kounis syndrome, 236 cases (47%) were suspectedly attributed to a single drug. Overall, 30 drugs emerged with disproportionality analysis, including non-steroidal anti-inflammatory drugs [e.g., ibuprofen, n=66; reporting odds ratio (ROR) =19.53; 95% confidence interval (CI), 11.4–24.2], antibiotics such as amoxicillin (n=29; ROR =16.6; 95% CI, 11.4–24.2) also in combination with clavulanate (n=45; ROR =198.42; 145.77–270.08), and anticancer drugs (fluorouracil: 10; ROR =4.29; 95% CI, 2.29–8.02). It is outlined that increased awareness among clinicians of drug-induced Kounis syndrome will allow early recognition, timely management, and final inclusion in treatment guidelines of myocardial infarction (80).
Variants
Three variants of Kounis syndrome have been described (19). The type I variant (coronary spasm) (17) constitutes a manifestation of endothelial dysfunction or microvascular angina. This group includes patients with normal, or nearly normal, coronary arteries, without predisposing factors for coronary artery disease. In these patients, acute release of inflammatory mediators can induce either coronary artery spasm without cardiac biomarkers, or coronary artery spasm progressing to acute myocardial infarction with raised cardiac biomarkers and troponins. The type II variant (80,81) refers to patients with quiescent pre-existing atheromatous disease, in whom the acute release of inflammatory mediators can induce either coronary artery spasm with normal cardiac biomarkers and troponins, or coronary artery spasm together with plaque erosion or rupture manifesting as acute myocardial infarction. The type III variant (82) includes patients with coronary artery stent thrombosis (subtype a), in whom aspirated thrombus specimens stained with hematoxylin-eosin and Giemsa demonstrated the presence of eosinophils and mast cells. Recently, a new subtype (subtype b) of this variant has been discovered, that includes patients with stent restenosis due to allergic inflammation (83-85). Both subtypes of variant III are also diagnosed in patients with stent implantation who died suddenly. Histological examination of the coronary intima or media and/or adventitia adjacent to stent deployment in these patients, showed infiltration by eosinophils and/or mast cells (86).
Causality
Drugs, environmental exposures, foods, and other conditions are the main triggers of Kounis syndrome. The most recently identified offenders of food-induced Kounis syndrome are fish, shellfish, milk, fruits, vegetables, and canned food. Histamine fish poisoning (scombroid syndrome), anisakiasis, caused by nematode parasite, and kiwifruit (Actinidia chinensis) allergy consist several characteristic causes of food-induced Kounis syndrome. Scombroid syndrome or histamine fish poisoning constitutes a histamine toxicity condition resulting from the consumption of spoiled fish (87). Fish flesh contains the amino acid histidine. When fish is infected by gram-negative bacteria containing the enzyme histidine decarboxylase, this enzyme converts histidine to histamine, and further induces Kounis syndrome. Anisakiasis is another condition associated with ingesting raw or undercooked fish or seafood. It occurs when seafood is infested with anisakis simplex (88), a common nematode parasitizing fish, that secretes allergenic substances. Therefore, unlike scombroid syndrome, anisakiasis is an IgE-mediated food allergy and future abstention from eating raw or undercooked fish or seafood is always required. In a recent report (89), a 75-year-old patient developed a type III variant of Kounis syndrome (stent thrombosis) following consumption of Greek rice pudding made of sheep milk, rice and sugar. The oral food challenge test revealed that the patient was allergic to sheep milk. Recently branched-chain amino acid supplements, used as energy source, were reported to have induced Kounis syndrome in a 17-year-old boy (90).
Overconsumption of medicines, hymenoptera exposures, climate and environmental conditions, pollen cross-reactivities, inadequacy of preventative measures and gene-environment interactions starting in early life constitute several Kounis syndrome causes. Indeed, a patient who had been admitted with chest pain to the emergency department, hospitalized for coronary vasospasm, and diagnosed with Kounis syndrome, was also diagnosed with heterozygous E148Q mutation (91). It is anticipated that with increased awareness of Kounis syndrome existence and further with the conduction of large prospective trials, the true incidence will be determined.
Diagnosis
Clinical symptoms and signs, together with laboratory, electrocardiographic, echocardiographic, and angiographic findings, help establishing a definite diagnosis. A high index of suspicion is of paramount importance in the diagnosis of Kounis syndrome. The newer diagnostic techniques such as cardiac magnetic resonance imaging (MRI) and myocardial scintigraphy can help confirming the diagnosis. Increased serum tryptase, histamine, cardiac biomarkers and cardiac troponins are particularly helpful findings. Measuring cardiac troponins in all patients admitted to the emergency department with acute allergic reactions, in order to timely diagnose and appropriately manage a potential cardiac injury manifesting as Kounis syndrome, has been suggested (61). Thallium-201 single-photon emission computer tomography (SPECT) and 125I-15-(p-iodophenyl)-3-(R,S) methyl pentadecanoic acid (BMIPP) SPECT have been used for investigating the type I variant of Kounis syndrome. These revealed severe myocardial ischemia, while coronary angiography showed normal coronary arteries (92). MRI with delayed contrast-enhanced images demonstrated normal washout in the sub-endocardial lesion area in patients with Kounis syndrome type I variant (93).
Conclusions
Differentiating myocardial tissue hypoperfusion caused by systemic vasodilation, plasma leakage due to vascular permeability leading to volume loss and reduced venous return from primary myocardial tissue damage and dysfunction due to coronary vasoconstriction and thrombosis induced by released anaphylactic mediators’ action (histamine, chymase, tryptase, cathepsin D, leukotrienes, thromboxane, platelet activating factor), remains seemingly challenging (94). In anaphylactic cardiovascular collapse, myocardial involvement due to vasospasm-induced coronary blood flow reduction manifesting as Kounis syndrome should be always considered (95). Clinically, combined treatment targeting the primary cause of anaphylaxis together with protection of cardiac tissue seems to be of paramount importance (96). More studies might be useful determine the importance of anaphylaxis mediators in myocardial pathobiology (97). Experimental studies combining contemporary measurements of peripheral arterial resistance and coronary blood flow with the newer diagnostic techniques namely cardiac MRI and myocardial scintigraphy, contributing further to the diagnosis and pathophysiology elucidation of anaphylactic cardiovascular collapse, are already in process.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Marone G, Genovese A, Varricchi G, et al. Human heart as a shock organ in anaphylaxis. Allergo J Int 2014;23:60-6. [Crossref] [PubMed]
- Bellou A, Al-Hammadi S, Aburawi EH, et al. 4-Aminopyridine, A Blocker of Voltage-Dependent K+ Channels, Restores Blood Pressure and Improves Survival in the Wistar Rat Model of Anaphylactic Shock. Crit Care Med 2016;44:e1082-9. [Crossref] [PubMed]
- Brown SG. The pathophysiology of shock in anaphylaxis. Immunol Allergy Clin North Am 2007;27:165-75. [Crossref] [PubMed]
- Matucci A, Vultaggio A, Fassio F, et al. Heart as the early main target of severe anaphylactic reactions: two case reports. Intern Emerg Med 2011;6:467-9. [Crossref] [PubMed]
- Low I, Stables S. Anaphylactic deaths in Auckland, New Zealand: a review of coronial autopsies from 1985 to 2005. Pathology 2006;38:328-32. [Crossref] [PubMed]
- Krombach JW, Kampe S, Keller CA, et al. Pharaoh Menes' death after an anaphylactic reaction--the end of a myth. Allergy 2004;59:1234-5. [Crossref] [PubMed]
- Wadell LA. Egyptian civilization it’s sumerian original and real chronology. London: Luzac & Co, 1930;60-3.
- Howell WH. Contributions to Medical Research, Dedicated to Victor Clarence Vaughan, Ann Arbor 1903;51.
- Auer J. Lethal cardiac anaphylaxis in the rabbit. J Exp Med 1911;14:476. [Crossref] [PubMed]
- Auer J, Lewis PA. The physiology of the immediate reaction of anaphylaxis in the guinea-pig. J Exp Med 1910;12:151. [Crossref] [PubMed]
- Clark E. Serum carditis: morphologic cardiac alterations in man associated with serum disease. J Am Med Assoc 1938;110:1098-100. [Crossref]
- Wadsworth GM, Brown CH. Serum reaction complicated by acute carditis. J Pediat 1940;17:801-5. [Crossref]
- Rich AR, Gregory JE. Experimental evidence that lesions with basic characteristics of rheumatic carditis can result from anaphylactic hypersensitivity. Bull Johns Hopkins Hosp 1943;73:239-64.
- Czickeli H. Contribution to the problem of the allergic etiology of angina pectoris and myocardial infarct. Klin Med Osterr Z Wiss Prakt Med 1950;5:364-7. [PubMed]
- Pfister CW, Plice SG. Acute myocardial infarction during a prolonged allergic reaction to penicillin. Am Heart J 1950;40:945-7. [Crossref] [PubMed]
- Zosin P, Miclea F, Munteanu M. Allergic myocardial infarction. Rum Med Rev 1965;19:26-8. [PubMed]
- Kounis NG, Zavras GM. Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract 1991;45:121-8. [PubMed]
- Zavras GM, Papadaki PJ, Kokkinis CE, et al. Kounis syndrome secondary to allergic reaction following shellfish ingestion. Int J Clin Pract 2003;57:622-4. [PubMed]
- Nikolaidis LA, Kounis NG, Gradman AH. Allergic angina and allergic myocardial infarction: a new twist on an old syndrome. Can J Cardiol 2002;18:508-11. [PubMed]
- Kovanen PT, Kaartinen M, Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation 1995;92:1084-8. [Crossref] [PubMed]
- Constantinides P. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation 1995;92:1083. [Crossref] [PubMed]
- Braunwald E. Unstable angina. An etiologic approach to management. Circulation 1998;98:2219-22. [Crossref] [PubMed]
- Kounis NG. Kounis syndrome (allergic angina and allergic myocardial infarction): a natural paradigm? Int J Cardiol 2006;110:7-14. [Crossref] [PubMed]
- Kounis NG, Kounis GN, Kouni SN, et al. Allergic reactions following implantation of drug eluting stents: a manifestation of Kounis syndrome? J Am Coll Cardiol 2006;48:592-3. [Crossref] [PubMed]
- Simons FE, Ardusso LR, Bilo MB, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J 2014;7:9. [Crossref] [PubMed]
- Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: Results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol 2010;126:798-806.e13. [Crossref] [PubMed]
- Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011;128:e9-17. [Crossref] [PubMed]
- Kay AB. Allergy and allergic diseases. Second of two parts. N Engl J Med 2001;344:109-13. [Crossref] [PubMed]
- Kirshbaum BA, Cohen HB, Beerman H, et al. The basophil degranulation test. A review of the literature. Am J Med Sci 1967;253:473-92. [PubMed]
- MacGlashan DW Jr, Bochner BS, Adelman DC, et al. Down-regulation of FceRI expression in human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol 1997;158:1438-45. [PubMed]
- Worm M, Edenharter G, Rueff F, et al. Symptom profile and risk factors of anaphylaxis in Central Europe. Allergy 2012;67:691-8. [Crossref] [PubMed]
- Pumphrey R. Anaphylaxis: can we tell who is at risk of a fatal reaction? Curr Opin Allergy Clin Immunol 2004;4:285-90. [Crossref] [PubMed]
- Kounis NG, Soufras GD, Hahalis G. Anaphylactic Shock: Kounis Hypersensitivity-Associated Syndrome Seems to be the Primary Cause. N Am J Med Sci 2013;5:631-6. [Crossref] [PubMed]
- Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med 2001;345:588-95. [Crossref] [PubMed]
- Levy JH, Adkinson NF Jr. Anaphylaxis during cardiac surgery: implications for clinicians. Anesth Analg 2008;106:392-403. [Crossref] [PubMed]
- Schummer W, Schummer C, Wipperman J, et al. Anaphylactic shock: Is vasopressin the drug of choice? Anesthesiology 2004;101:1025-7. [Crossref] [PubMed]
- Fisher MM. Clinical observations on the pathophysiology and treatment of anaphylactic cardiovascular collapse. Anaesth Intensive Care 1986;14:17-21. [PubMed]
- Fahmy NR. Hemodynamics, plasma histamine and catecholamine concentrations during an anaphylactoid reaction to morphine. Anesthesiology 1981;55:329-31. [Crossref] [PubMed]
- van der Linden PW, Struyvenberg A, Kraaijenhagen RJ, et al. Anaphylactic shock after insect-sting challenge in 138 persons with a previous insect-sting reaction. Ann intern Med 1993;118:161-8. [Crossref] [PubMed]
- Hanashiro PK, Weil MH. Anaphylactic shock in man: report of two cases with detailed hemodynamics and metabolic studies. Arch Intern Med 1967;119:129-40. [Crossref] [PubMed]
- Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539-48. [Crossref] [PubMed]
- Brown SG. Cardiovascular aspects of anaphylaxis: Implications for treatment and diagnosis. Curr Opin Allergy Clin Immunol 2005;5:359-64. [Crossref] [PubMed]
- Wallén NH, Held C, Rehnqvist N, et al. Effects of mental and physical stress on platelet function in patients with stable angina pectoris and healthy controls. Eur Heart J 1997;18:807-15. [Crossref] [PubMed]
- Dell'Italia LJ, Collawn JF, Ferrario CM. Multifunctional Role of Chymase in Acute and Chronic Tissue Injury and Remodeling. Circ Res 2018;122:319-36. [Crossref] [PubMed]
- Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol 2017;140:335-48. [Crossref] [PubMed]
- Liu M, Yokomizo T. The role of leukotrienes in allergic diseases. Allergol Int 2015;64:17-26. [Crossref] [PubMed]
- Smyth EM. Thromboxane and the thromboxane receptor in cardiovascular disease. Clin Lipidol 2010;5:209-19. [Crossref] [PubMed]
- Wacker MJ, Kosloski LM, Gilbert WJ, et al. Inhibition of thromboxane A2-induced arrhythmias and intracellular calcium changes in cardiac myocytes by blockade of the inositol trisphosphate pathway. J Pharmacol Exp Ther 2009;331:917-24. [Crossref] [PubMed]
- Zavecz JH, Levi R. Separation of primary and secondary cardiovascular events in systemic anaphylaxis. Circ Res 1977;40:15-19. [Crossref] [PubMed]
- Levi R. Cardiac anaphylaxis: models, mediators, mechanisms, and clinical considerations. In: Marone G, Lichtenstein LM, Condorelli M, Fauci AS, editors. Human inflammatory disease, clinical immunology. Toronto, Canada: Decker, 1988;93-105.
- Tosaki A, Koltai M, Braquet P, et al. Possible involvement of platelet activating factor in anaphylaxis of passively sensitised, isolated guinea pig hearts. Cardiovasc Res 1989;23:715-22. [Crossref] [PubMed]
- Felix SB, Baumann G, Berdel WE. Systemic anaphylaxis—separation of cardiac reactions from respiratory and peripheral vascular events. Res Exp Med (Berl) 1990;190:239-52. [Crossref] [PubMed]
- Zhang W, Shibamoto T, Tanida M, et al. Rat hepatic and splanchnic vascular responses to anaphylactic shock, compared with hemorrhagic or vasodilator-induced shock. In Vivo 2013;27:485-93. [PubMed]
- Cui S, Shibamoto T, Takano H, et al. Leukotrienes and cyclooxygenase products mediate anaphylactic venoconstriction in ovalbumin sensitized rat livers. Eur J Pharmacol 2007;576:99-106. [Crossref] [PubMed]
- Davidson J, Zheng F, Tajima K, et al. Anaphylactic shock decreases cerebral blood flow more than what would be expected from severe arterial hypotension. Shock 2012;38:429-35. [Crossref] [PubMed]
- Kounis N, Kounis GN, Soufras GD, et al. Anaphylactic shock decreases cerebral blood flow more than what would be expected from severe arterial hypotension. Shock 2013;39:462-3. [Crossref] [PubMed]
- Kuda Y, Kurata Y, Wang M, et al. Major contribution of vasospasm-induced coronary blood flow reduction to anaphylactic ventricular dysfunction assessed in isolated blood-perfused rat heart. Cardiol J 2014;21:11-7. [PubMed]
- Kounis NG, Soufras GD. Kounis syndrome: A primary cause for the anaphylactic shock. Cardiol J 2014;21:102-3. [Crossref] [PubMed]
- Wang M, Shibamoto T, Tanida M, et al. Mouse anaphylactic shock is caused by reduced cardiac output, but not by systemic vasodilatation or pulmonary vasoconstriction, via PAF and histamine. Life Sci 2014;116:98-105. [Crossref] [PubMed]
- Milicic V, Zivkovic V, Jeremic N, et al. Coronary flow and oxidative stress during local anaphylactic reaction in isolated mice heart: the role of nitric oxide (NO). Mol Cell Biochem 2016;412:221-7. [Crossref] [PubMed]
- Lippi G, Buonocore R, Schirosa F, et al. Cardiac troponin I is increased in patients admitted to the emergency department with severe allergic reactions. A case-control study. Int J Cardiol 2015;194:68-9. [Crossref] [PubMed]
- Kounis NG, Mazarakis A, Bardousis C, et al. The heart and coronary arteries as primary target in severe allergic reactions: Cardiac troponins and the Kounis hypersensitivity-associated acute coronary syndrome. Int J Cardiol 2015;198:83-4. [Crossref] [PubMed]
- Cha YS, Kim H, Bang MH, et al. Evaluation of myocardial injury through serum troponin I and echocardiography in anaphylaxis. Am J Emerg Med 2016;34:140-4. [Crossref] [PubMed]
- Gangadharan V, Bhatheja S, Al Balbissi K. Kounis syndrome-An atopic monster for the heart. Cardiovasc Diagn Ther 2013;3:47-51. [PubMed]
- Mukta V, Chandragiri S, Das AK. Allergic myocardial infarction. N Am J Med Sci 2013;5:157-8. [Crossref] [PubMed]
- Kajander OA, Virtanen MP, Sclarovsky S, et al. Iatrogenic inverted takotsubo syndrome following intravenous adrenaline injections for an allergic reaction. Int J Cardiol 2013;165:e3-5. [Crossref] [PubMed]
- Shah G, Scadding G, Nguyen-Lu N, et al. Peri-operative cardiac arrest with ST elevation secondary to gelofusin anaphylaxis—Kounis syndrome in the anaesthetic room. Int J Cardiol 2013;164:e22-6. [Crossref] [PubMed]
- Satish R, Kanchan R, Yashawant R, et al. Acute myocardial infarction in a stented patient following snake bite-possibility of stent thrombosis—a case report. Indian Heart J 2013;65:327-30. [Crossref] [PubMed]
- Kounis NG, Mazarakis A, Bardousis C. Myocardial injury through serum troponin I and echocardiography in anaphylaxis: Takotsubo cardiomyopathy and the Kounis hypersensitivity-associated acute coronary syndrome. Am J Emerg Med 2016;34:650-1. [Crossref] [PubMed]
- Triggiani M, Montagni M, Parente R, et al. Anaphylaxis and cardiovascular diseases: a dangerous liaison. Curr Opin Allergy Clin Immunol 2014;14:309-15. [Crossref] [PubMed]
- Kounis NG. Coronary hypersensitivity disorder: the Kounis syndrome. Clin Ther 2013;35:563-71. [Crossref] [PubMed]
- Hasegawa S, Tashiro N, Matsubara T, et al. A comparison of FcepsilonRI-mediated RANTES release from human platelets between allergic patients and healthy individuals. Int Arch Allergy Immunol 2001;125:42-7. [Crossref] [PubMed]
- Kounis NG, Mazarakis A, Tsigkas G, et al. Kounis syndrome: a new twist on an old disease. Future Cardiol 2011;7:805-24. [Crossref] [PubMed]
- Akoz A, Tanboga HI, Emet M, et al. A prospective study of Kounis syndrome: Clinical experience and cardiac magnetic resonance imaging findings for 21 patients. Acta Med Mediterraea 2013;9:811-6.
- Renda F, Landoni G, Trotta F, et al. Kounis Syndrome: An analysis of spontaneous reports from international pharmacovigilance database. Int J Cardiol 2016;203:217-20. [Crossref] [PubMed]
- Abdelghany M, Subedi R, Shah S, et al. Kounis syndrome: A review article on epidemiology, diagnostic findings, management and complications of allergic acute coronary syndrome. Int J Cardiol 2017;232:1-4. [Crossref] [PubMed]
- Ueno M, Yanagawa Y, Omori K, et al. Kounis syndrome induced by contrast medium. CHUBU J Jpn Assoc Acute Med 2014;10:11-3.
- Yanagawa Y, Oode Y, Kunimoto M, et al. A case of Kounis syndrome induced by food allergies. Sch J Med Case Rep 2015;9A:834-7.
- Yanagawa Y, Kondo A, Ishikawa K, et al. Kounis syndrome should be excluded when physicians treat patients with anaphylaxis. Ann Allergy Asthma Immunol 2017;119:392. [Crossref] [PubMed]
- Raschi E, Fertonani Affini L, Antonazzo IC, et al. Drug-induced Kounis syndrome: A matter of pharmacovigilance. Int J Cardiol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Tsigkas G, Chouchoulis K, Kounis NG, et al. Allergic reaction reveals a non-lethal late stent thrombosis. A new subtype of Kounis syndrome? Int J Cardiol 2011;149:281-2. [Crossref] [PubMed]
- Biteker M. A new classification of Kounis syndrome. Int J Cardiol 2010;145:553. [Crossref] [PubMed]
- Kounis NG, Koniari I, Roumeliotis A, et al. Thrombotic responses to coronary stents, bioresorbable scaffolds and the Kounis hypersensitivity-associated acute thrombotic syndrome. J Thorac Dis 2017;9:1155-64. [Crossref] [PubMed]
- Itoh T, Nakajima Y, Morino Y. Proposed classification for a variant of Kounis syndrome. Clin Chem Lab Med 2017;55. [Crossref] [PubMed]
- Itoh T, Nakajima Y, Morino Y, et al. Kounis syndrome: New classification. Int J Cardiol 2018;256:11. [Crossref] [PubMed]
- Kounis NG. Natural Paradigm: Kounis Hypersensitivity associated Acute Coronary Syndrome. Ach Iatrike 2016;35:16-21.
- Kounis NG, Patsouras N, Grapsas N, et al. Histamine induced coronary artery spasm, fish consumption and Kounis syndrome. Int J Cardiol 2015;193:39-41. [Crossref] [PubMed]
- Pravettoni V, Primavesi L, Piantanida M. Anisakis simplex: current knowledge. Eur Ann Allergy Clin Immunol 2012;44:150-6. [PubMed]
- Tzanis G, Bonou M, Mikos N, et al. Early stent thrombosis secondary to food allergic reaction: Kounis syndrome following rice pudding ingestion. World J Cardiol 2017;9:283-8. [Crossref] [PubMed]
- Dogan V. A case of Kounis syndrome associated with branched-chain amino acid supplementation in a 17-year-old boy. Cardiol Young 2018;6:1-3. [Crossref] [PubMed]
- Saylan B, Cevik A, Firat C. Kounis syndrome, a cause of chest pain to keep in mind, may be associated with E148Q mutation. Hong Kong J Emerg 2012;19:278-82. [Crossref]
- Goto K, Kasama S, Sato M, et al. Myocardial scintigraphic evidence of Kounis syndrome: what is the aetiology of acute coronary syndrome? Eur Heart J 2016;37:1157. [Crossref] [PubMed]
- Okur A, Kantarci M, Karaca L, et al. The utility of cardiac magnetic resonance imaging in Kounis syndrome. Postepy Kardiol Interwencyjnej 2015;11:218-23. [Crossref] [PubMed]
- Kounis NG, Davlouros P, Hahalis G, et al. The heart seems to be the primary site and the target of anaphylaxis resulting in the development of Kounis syndrome. Intern Emerg Med 2012;7 Suppl 2:S119-20. [Crossref] [PubMed]
- Kounis N, Kounis G. Anaphylactic cardiovascular collapse during anesthesia: the Kounis acute hypersensitivity syndrome seems to be the most likely cause. J Korean Med Sci 2013;28:638-9. [Crossref] [PubMed]
- Kounis NG, Soufras GD, Davlouros P, et al. Combined etiology of anaphylactic cardiogenic shock: amiodarone, epinephrine, cardioverter defibrillator, left ventricular assist devices and the Kounis syndrome. Ann Card Anaesth 2015;18:261-4. [Crossref] [PubMed]
- Kounis NG, Koniari I, Soufras G, et al. Targeted temperature management in Kounis syndrome following cardiac arrest with anaphylaxis. Am J Emerg Med 2018;36:727-8. [Crossref] [PubMed]


