Evaluation of cardiac function using heart-lung interactions
Introduction
The existence of an interdependence between the cardiovascular and respiratory systems has long been recognised and described by physiologists. As early as the 1700s, Stephen Hales, successfully cannulated the carotid artery of a horse and described cyclical variation in the height of blood within a glass tube with respiration (1). Whilst Valsalva described expiration against a closed glottis leading to temporary cessation of cardiac output (CO) in 1740, it was not until the 1900s that this effect was further described and the changes were attributed to changes in intrathoracic pressure (ITP) (2).
With increased understanding of the physiology underpinning heart-lung interactions, methods of applying these interactions to evaluate an individual patient’s cardiac function have been devised. One example of their use is as part of an assessment of a patient’s fluid status and for prediction of fluid responsiveness. Alternatively difficulty with respiratory weaning may reflect underlying cardiac dysfunction.
Why assess cardiac function?
Understanding a patient’s cardiac function is vital to individualise their treatment and management in critical care. Adequate cardiac function is required to maintain end-organ perfusion and deliver sufficient oxygen to the tissues. In a haemorrhagic model with evidence of tissue hypoperfusion, intravenous fluid can be given to increase cardiac preload, increase CO and restore tissue perfusion (3).
A patient is labeled as a fluid responder if he or she increases their stroke volume or CO by more than 10–15% in response to a fluid challenge. A fluid challenge is a small amount of fluid given intravenously over a short period of time (4). The difficulty is in deciding who to give intravenous fluids to, given that only about 50% of patients admitted to intensive care units are fluid responders (5). Hence, the prediction of fluid responsiveness has been a topic of great interest in recent years. Fluid responsiveness refers to the ability of the ventricles to increase the volume ejected, in response to an increase in preload. Evaluation of heart lung interactions can help predict those patients that may be fluid responsive, prior to administering any fluid and potentially reduce the amount of fluid given to non-responders. The use of these dynamic markers in patients with shock has been recommended by the 2004 consensus statement (6).
The utility of heart lung interactions extends beyond just assessment of fluid responsiveness and can provide information about both left and right heart function. A failure to wean from the ventilator may reflect poor cardiac function rather than a true respiratory problem, even if the patient was initially intubated for primary respiratory failure. Assessment of the patient’s cardiac function may help guide whether these patients will benefit from diuresis to decrease preload, or vasodilators to reduce afterload (7).
Physiology of heart lung interactions
The Frank Starling mechanism explains how the heart is able to accommodate then eject all the blood returned to it, despite variations in venous return. In a normal functioning heart, when preload increases, this increases stretch of the myocytes, increasing the end-diastolic volume. This increases the strength of ventricular contraction and the volume of blood ejected from the ventricle (8).
The rate of blood flow is determined by the difference in pressure between two points in the cardiovascular system. Given the large proportion of blood contained within the venous reservoir, the pressure there is particularly interesting. According to Guyton, venous return is defined by three parameters: the mean systemic filling pressure (Pmsf), the right atrial pressure (RAP) and the resistance to venous return (RVR). This can be mathematically represented as follows:
VR = (Pmsf−RAP)/RVR
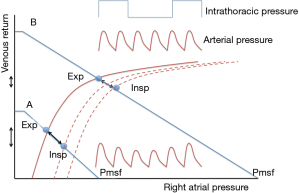
Pmsf is the pressure measured throughout the circulatory system when there is no blood motion, and it is considered the driving pressure of venous return. Pmsf depends on the mean capacity of the system and the intravascular stressed volume, which in turns depends on the total intravascular volume. The pressure gradient between Pmsf and RAP is directly proportional to venous return. Guyton described venous return curves and changes in the RAP under isovolumetric conditions. The higher the RAP, the lower the venous return (9,10). During steady conditions CO must equal venous return, therefore the gradient between Pmsf and RAP is a key determinant of CO.
ITP is defined as the pressure within the pleural cavity. During spontaneous ventilation, ITP decreases during inspiration and becomes negative and then increases again with expiration. The opposite effect is seen during mechanical ventilation, ITP increases with inspiration (11). Inspiration in spontaneous ventilation is an active process involving contraction of the respiratory muscles, whereas during positive pressure ventilation the increased airway pressure results in passive expansion of the lungs (12).
The relationship between ITP, venous return and cardiac function forms the basis for understanding heart lung interactions. In a patient receiving positive pressure ventilation, in inspiration there is an increase in ITP and RAP. As venous return is reliant on a pressure gradient, it decreases during inspiration exactly as Guyton demonstrated in his experiments (1). The decrease in venous return in inspiration decreases right ventricular filling and subsequently the output from the right ventricle falls. In addition, increased right ventricular afterload compounds this effect, secondary to the effect of increased transpulmonary pressure from positive pressure ventilation. The effects of this are seen on the left ventricle during expiration, due to pulmonary transit time. The pulmonary transit time means that the effects of the change in ITP on the left ventricle are mirrored in the right ventricle but at the opposite phase of the respiratory cycle. Therefore, during expiration we observe a decrease in left ventricular preload and decreased output from the left ventricle, or in other words a decreased stroke volume (13).
The effect of decreased right then left ventricular preload, and subsequently decreased left ventricular stroke volume is more marked when the heart is working on the steep part of the Frank Starling curve, as demonstrated in Figure 1. The magnitude of the cardiovascular response to the cyclic changes of ITP may be used to assess where on the Frank Starling curve the patient is working. This will provide information about cardiac function at that time point.
A number of our methods of making a clinical assessment of a patient’s cardiac function and whether they may benefit from additional fluid rely on these interactions. These include pulse pressure variation (PPV), stroke volume variation (SVV), dynamic arterial elastance, end expiratory occlusion test and use of echocardiographic parameters. These tests will be further discussed but primarily work by altering preload.
Methods of evaluation of cardiac function
SVV and PPV
SVV and PPV can be used to provide information about cardiac function in ventilated patients. The concept was developed following initial studies by Perel et al. that stated systolic pressure variation was an indicator of hypovolemia in animal models (14).
Pulse pressure (PP) is the difference between systolic and diastolic blood pressure and PPV can be calculated using the formula: PPV = (PPmax − PPmin)/[(PPmax + PPmin)/2]. One systematic review and meta-analysis showed that the diagnostic accuracy was greater for PPV than SVV. SVV is based on measurements calculated from pulse contour or pulse power analysis. A PPV of more than 11–13% has been shown to be a predictor of fluid responsiveness (15).
Use of PPV and SVV is limited by a number of preconditions that must be met. The patient must be fully ventilated with a tidal volume of 8 mL/kg, which is more than the lung protective ventilation strategy generally employed. It can also not be used in patients with cardiac arrhythmias (16). These are likely some of the reasons that despite its simplicity as a test, it is not frequently used by clinicians (17).
Dynamic arterial elastance
Dynamic arterial elastance (Eadyn) is a measure of arterial tone and can be calculated using the PPV to SVV ratio (18). It can be used to predict the change in arterial pressure in response to a fluid challenge, in hypotensive patients who are fluid responsive. One study showed that an Eadyn of 0.89 or more was able to predict which patients would respond to fluid with an increase in mean arterial pressure, with a sensitivity of 94% and a specificity of 100%. The authors concluded that use of Eadyn may help differentiate hypotensive patients who will benefit from fluid alone, versus those who also require early vasopressors (19,20).
End expiratory occlusion test
A short end expiratory occlusion, performed during mechanical ventilation has been shown to be another method of testing fluid responsiveness, without giving a fluid challenge. Its advantage over SVV or PPV is that it can be used in patients with cardiac arrhythmias and ventilated patients with some spontaneous respiratory effort. This difference exists as the effect of the end expiratory occlusion test is assessed over several cardiac cycles (21). An increase in CO of more than 5% in response to the end expiratory occlusion test is predictive of fluid responsiveness (22).
This test also utilizes the heart lung interactions previously described. The end expiratory occlusion, by definition prevents inspiration and thereby prevents the associated rise in ITP, which would have decreased venous return. The increase in transpulmonary pressure associated with inspiration will also not occur. Following an end expiratory occlusion test, venous return increases, increasing right ventricular preload. If the patient is volume responsive this will result in an increased right ventricular stroke volume. Subsequently left ventricular preload increases and the increase in left ventricular output, or CO can be measured (22).
Inferior and superior vena cava (SVC) diameter changes
An alternative method of assessing cardiac function is the use of echocardiography to directly image the heart and major vessels. Some aspects of this assessment utilize the effects of respiratory variations, or heart lung interactions. Echocardiography can be used to assess changes with respiration in the diameter of either the superior or inferior vena cava (IVC), or alternatively changes in aortic flow velocity can be measured (1).
During positive pressure ventilation, the IVC diameter is maximal during inspiration. The change in diameter with the respiratory cycle can be used to calculate the IVC distensibility index. However one study showed that the change in IVC could only be successfully measured in 78% of patients studied (23). Similarly change in the diameter of the SVC can be measured. This requires transesophageal echocardiography but is a more specific predictor of fluid responsiveness (23). The threshold for IVC distensibility index has been demonstrated to be 18% (24). For SVC diameter, a change of more than 36% with respiration, has been shown to be predictive of fluid responsiveness (25).
Variation of aortic blood flow velocity
Changes in aortic blood flow velocity were shown to be the most sensitive measurement for fluid responsiveness, when compared to changes in IVC and SVC diameter (23). Pulsed Doppler waves of aortic blood flow during the respiratory cycle are obtained with transthoracic echocardiography. The maximum and minimum peak aortic blood flow velocities (Vpeak max and Vpeak min) were measured. ΔVpeak was calculated as ΔVpeak (%)=100× (Vpeak max − Vpeak min)/[(Vpeak max + Vpeak min)/2]. A study performed in thirty-three mechanically ventilated children, showed a ΔVpeak at a cut off value of 11% can be used to predict fluid responsiveness and was a better predictor than both SVV and PVV (26).
Plethysmographic variability index (PVI)
PPV and stroke volume variation require invasive measurement of arterial pressure. The pulse oximetry plethysmographic (POP) waveform depends on arterial pulsatility and stroke volume. Canneson et al. demonstrated that there was a strong correlation and a good agreement between respiratory variation in arterial PP and respiratory variation in POP waveform amplitude. Several studies have since shown that the respiratory changes in the amplitude of the POP pulse wave are as accurate as PPV for predicting fluid responsiveness in mechanically ventilated patients with a cut off of around 14–17% (27-29). As a result, a commercial device was designed for providing an automatic calculation of the respiratory variation of the POP signal, called “pleth variability index” (PVI), and this index seems to be reliable to predict fluid responsiveness in perioperative settings (30-33), but not so reliable in patients requiring noradrenaline (34).
Continuous positive airway pressure (CPAP)
CPAP is a commonly used treatment option in pulmonary oedema secondary to cardiac failure. CPAP has an effect on both cardiac preload and afterload; the resultant clinical change seen in response provides information about the underlying cardiac function (35). In general it decreases RV preload and LV afterload. In a normal heart, the cardiac function is predominately dependent on preload, so the decrease in preload with initiation of CPAP decreases CO. This effect will be more marked in those who are hypovolemic. In left ventricular failure the effect on afterload is beneficial, and an increase in CO is seen. Broadly speaking patients with cardiac failure are generally hypervolemic and as such the effect on RV preload is well tolerated (36).
These changes are explained by the following effects, caused by the initiation of CPAP: an increase in ITP and an effect on pulmonary vascular resistance (PVR) secondary to change in lung volume and a decrease in LV transmural pressure (35). The effect of increased ITP and the resultant decrease in venous return has already been explained. This is the mechanism for the decrease in RV preload that is seen with CPAP.
Systolic transmural pressure is a key determinant of LV afterload. It has been shown that the decrease in transmural pressure following application of CPAP is greater in those with cardiac failure when compared to healthy volunteers. In one study oesophageal pressure was used as a marker of extracardiac pressure. CPAP was shown to increase oesophageal pressure in a dose dependent fashion with resultant decrease in transmural pressure in those with cardiac failure. In the healthy volunteers a much smaller effect was seen on oesophageal pressure and this was offset by an increase systolic blood pressure with a lack of overall effect on transmural pressure seen in the healthy volunteers (37).
Acute respiratory distress syndrome (ARDS)
One frequently encountered clinical application of heart lung interactions is in the assessment of a patient’s cardiac function, in the presence of ARDS. In ARDS the lung has diffuse changes, with increased permeability and oedema, resulting in increased lung mass (38). This results in compression of both the vasculature and alveoli. Positive end expiratory pressure (PEEP) is used to try to improve alveoli recruitment. The PEEP may be expected to negatively impact on CO due to an impairment of venous return and therefore CO. However, it may act to decrease right ventricular afterload and thereby increase CO, through two main effects on PVR (36). In ARDS significant hypoxia may result in hypoxic pulmonary vasoconstriction (39). One aim of increased PEEP, is improved alveoli recruitment and subsequent reversal of the hypoxic pulmonary vasoconstriction and a decrease in PVR with a resultant increase in pulmonary vascular capacitance. PVR also alters with a change in lung volume, with the lowest PVR seen at functional residual capacity (FRC), and highest at residual volume and total lung capacity (40). Optimal PEEP, influences lung volume and may therefore help lower PVR. This offloads the RV and improves LV function, maintaining CO. Through assessment of changes in CO with titration of PEEP information about biventricular, but in particular right heart function, can be obtained (36).
Weaning failure
Difficulty in weaning a patient from a ventilator is another clinical scenario where the heart lung interactions can provide information about underlying cardiac function. When a patient is weaned from the ventilator, the degree of mechanical support is reduced and the work of breathing is returned to the patient. There is a change from positive pressure ventilation to negative pressure ventilation. This decreases ITP, increasing the gradient for venous return and results in increased preload. LV afterload is increased secondary to the decreased ITP and increased systemic arterial pressure. Finally RV afterload may also increase, in the setting of increased pulmonary vasculature capacitance with decreased ITP (7,41).
If the cardiac system is unable to compensate for these changes, then the patient may fail the weaning trial. This has been associated with increase in left ventricular end diastolic pressure (LVEDP) and left ventricular dysfunction. An understanding of heart lung interactions can raise suspicion of a likely cardiac cause of the failure to wean and prompt further investigations. Once diagnosed, optimization of cardiac function and fluid management, increases the success of subsequent trials (41,42).
Conclusions
The interactions between ITP and the blood flow through the cardiac chambers during a respiratory cycle, provides an opportunity to obtain basic information regarding a patient’s cardiac function. SVV, PPV, dynamic arterial elastance, end-expiratory occlusion test, assessment of superior or IVC diameter, variation of aortic blood flow velocity and PVI are all methods that use this interaction to situate the patient in a particular area of the Frank-Starling curve and eventually predict fluid responsiveness.
An understanding of the interplay between the cardiac and respiratory systems is also important in the everyday management of critically ill patients. This includes the use of CPAP in left ventricular failure and prediction of the haemodynamic effects of ventilation in ARDS.
Acknowledgements
None.
Footnote
Conflicts of Interest: M Cecconi declares the following conflicts of interest: consultancy for Edwards Lifesciences, Cheetah Medical, LiDCO and Directed Systems. Other authors have no conflicts of interest to declare.
References
- Grübler MR, Olivier W, Berger D, et al. Basic concepts of heart-lung interactions during mechanical ventilation. Swiss Med Wkly 2017;147. [PubMed]
- Barr J. The effects of respiration on the circulation. Br Med J 1907;1:913-8. [Crossref] [PubMed]
- Gruartmoner G, Mesquida J, Ince C. Fluid therapy and the hypovolemic microcirculation. Curr Opin Crit Care 2015;21:276-84. [Crossref] [PubMed]
- Cecconi M, Parsons AK, Rhodes A. What is a fluid challenge? Curr Opin Crit Care 2011;17:290-5. [Crossref] [PubMed]
- Michard F, Teboul JL. Predicting Fluid Responsiveness in ICU Patients. Chest 2002;121:2000-8. [Crossref] [PubMed]
- Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1795-815. [Crossref] [PubMed]
- Teboul JL, Monnet X, Richard C. Weaning failure of cardiac origin: Recent advances. Crit Care 2010;14:211. [Crossref] [PubMed]
- Patterson SW, Piper H, Starling EH. The regulation of the heart beat. J Physiol 1914;48:465-513. [Crossref] [PubMed]
- Guyton AC. Regulation of cardiac output. Anesthesiology 1968;29:314-26. [Crossref] [PubMed]
- Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev 1955;35:123-9. [Crossref] [PubMed]
- Wise RA, Robotham JL, Summer WR. Effects of spontaneous ventilation on the circulation. Lung 1981;159:175-86. [PubMed]
- Pinsky MR. Cardiovascular issues in respiratory care. Chest 2005;128:592S-7S. [Crossref] [PubMed]
- Michard F, Boussat S, Chemla D, et al. Relation between Respiratory Changes in Arterial Pulse Pressure and Fluid Responsiveness in Septic. Am J Respir Crit Care Med 2000;162:134-8. [Crossref] [PubMed]
- Perel A, Pizov R, Cotev S. Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology 1987;67:498-502. [Crossref] [PubMed]
- Marik PE, Cavallazzi R, Vasu T, et al. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: A systematic review of the literature*. Crit Care Med 2009;37:2642-7. [Crossref] [PubMed]
- De Backer D, Heenen S, Piagnerelli M, et al. Pulse pressure variations to predict fluid responsiveness: Influence of tidal volume. Intensive Care Med 2005;31:517-23. [Crossref] [PubMed]
- Cecconi M, Hofer C, Teboul JL, et al. Fluid challenges in intensive care: the FENICE study: A global inception cohort study. Intensive Care Med 2015;41:1529-37. [Crossref] [PubMed]
- Pinsky MR. Protocolized cardiovascular management based on ventricular-arterial coupling. Funct hemodynamic Monit 2006;381-95.
- García MI, Cano AG, Romero MG. Dynamic arterial elastance to predict arterial pressure response to volume loading in preload-dependent patients. Crit Care 2011;15:R15. [PubMed]
- García MI, Romero MG, Cano AG, et al. Dynamic arterial elastance as a predictor of arterial pressure response to fluid administration: A validation study. Crit Care 2014;18:626. [Crossref] [PubMed]
- Monnet X, Teboul JL. Assessment of volume responsiveness during mechanical ventilation: Recent advances. Crit Care 2013;17:217. [PubMed]
- Monnet X, Osman D, Ridel C, et al. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med 2009;37:951-6. [Crossref] [PubMed]
- Vignon P, Repessé X, Begot E, et al. Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med 2017;195:1022-32. [Crossref] [PubMed]
- Barbier C, Loubières Y, Schmit C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med 2004;30:1740-6. [Crossref] [PubMed]
- Vieillard-Baron A, Chergui K, Rabiller A, et al. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med 2004;30:1734-9. [Crossref] [PubMed]
- Byon HJ, Lim CW, Lee JH, et al. Prediction of fluid responsiveness in mechanically ventilated children undergoing neurosurgery. Br J Anaesth 2013;110:586-91. [Crossref] [PubMed]
- Wyffels PA, Durnez PJ, Helderweirt J, et al. Ventilation-induced plethysmographic variations predict fluid responsiveness in ventilated postoperative cardiac surgery patients. Anesth Analg 2007;105:448-52. [Crossref] [PubMed]
- Feissel M, Teboul JL, Merlani P, et al. Plethysmographic dynamic indices predict fluid responsiveness in septic ventilated patients. Intensive Care Med 2007;33:993-9. [Crossref] [PubMed]
- Cannesson M, Attof Y, Rosamel P, et al. Respiratory variations in pulse oximetry plethysmographic waveform amplitude to predict fluid responsiveness in the operating room. Anesthesiology 2007;106:1105-11. [Crossref] [PubMed]
- Loupec T, Nanadoumgar H, Frasca D, et al. Pleth variability index predicts fluid responsiveness in critically ill patients. Crit Care Med 2011;39:294-9. [Crossref] [PubMed]
- Hood JA, Wilson RJ. Pleth variability index to predict fluid responsiveness in colorectal surgery. Anesth Analg 2011;113:1058-63. [Crossref] [PubMed]
- Zimmermann M, Feibicke T, Keyl C, et al. Accuracy of stroke volume variation compared with pleth variability index to predict fluid responsiveness in mechanically ventilated patients undergoing major surgery. Eur J Anaesthesiol 2010;27:555-61. [PubMed]
- Cannesson M, Desebbe O, Rosamel P, et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth 2008;101:200-6. [Crossref] [PubMed]
- Monnet X, Guérin L, Jozwiak M, et al. Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. Br J Anaesth 2013;110:207-13. [Crossref] [PubMed]
- Kato T, Sudo S, Kasai T. Positive airway pressure therapy for heart failure. World J Cardiol 2014;6:1175-91. [Crossref] [PubMed]
- Luecke T, Pelosi P. Clinical review: Positive end-expiratory pressure and cardiac output. Crit Care 2005;9:607-21. [Crossref] [PubMed]
- Naughton MT, Rahman MA, Hara K, et al. Effect of Continuous Positive Airway Pressure on Intrathoracic and Left Ventricular Transmural Pressures in Patients With Congestive Heart Failure. Circulation 1995;91:1725-31. [Crossref] [PubMed]
- Gattinoni L, Tonetti T, Quintel M. Regional physiology of ARDS. Crit Care 2017;21:312. [Crossref] [PubMed]
- Marshall BE, Hanson CW, Frasch F, et al. Role of hypoxic pulmonary vasoconstriction in pulmonary gas exchange and blood flow distribution - 2. Pathophysiology. Intensive Care Med 1994;20:379-89. [Crossref] [PubMed]
- Simmons DH, Linde LM, Miller JH, et al. Relation Between Lung Volume and Pulmonary Vascular Resistance. Circ Res 1961;9:465-71. [Crossref]
- Dres M, Teboul JL, Monnet X. Weaning the cardiac patient from mechanical ventilation. Curr Opin Crit Care 2014;20:493-8. [Crossref] [PubMed]
- Lemaire F, Teboul JL, Cinotti L, et al. Acute Left Ventricular Dysfunction during Unsuccessful Weaning from Mechanical Ventilation. Anesthesiology 1988;69:171-9. [Crossref] [PubMed]


