Autologous platelet-rich gel combined with in vitro amplification of bone marrow mesenchymal stem cell transplantation to treat the diabetic foot ulcer: a case report
Introduction
Diabetic foot disease is a common chronic complication of diabetes. Diabetic neuropathy, peripheral vascular disease, secondary infection of the lower limbs, ulcers and/or deep tissue damage are the characteristics and bases of diabetic foot disease. Diabetic foot disease underlies the high rates of disability and mortality and the large burden of medical expenses. The average prevalence rate of diabetic foot disease in China is 5.7%, and age, disease duration, patient weight, smoking, hypertension and retinopathy are all contributing factors to diabetic foot disease. Diabetic patients have been estimated to have a lifetime risk of 15% for developing a foot ulcer. Because the diabetic foot ulcer (DFU) is a common occurrence and because traditional treatment (including the control of blood glucose, blood lipids, and blood pressure; debridement; dressing; circulation improvement with drugs such as alprostadil; anti-infection treatment with sensitive antibiotics; anti-platelet treatment; lower limb interventional surgery; continuous negative pressure suction; off-loading; and other therapy) is ineffective, the risk of lower limb amputation is 40 times higher for diabetic patients than for non-diabetic patients, and approximately 85% of amputations are caused by DFUs (1). There is an urgent need for more effective interventions for the DFU in clinical practice. We report a case of a diabetic patient who presented at the hospital for a routine follow-up visit with a complaint of an infected ulcer in her right foot. After receiving the abovementioned traditional treatment, she was successfully treated with the autologous platelet-rich gel (APG) and in vitro amplification of bone marrow mesenchymal stem cell (BMMSC) transplantation.
Case presentation
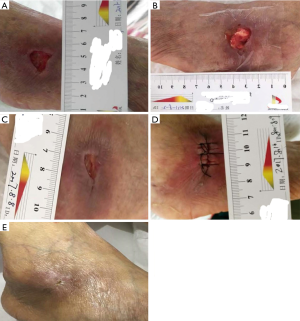
A 54-year-old Chinese female with a medical history of diabetes over the previous 10 years without monitoring of the blood glucose was enrolled. She presented to our hospital with complaints of a progressively worsening infection of the right foot ulcer for over 15 days on 5 July 2017 after using traditional Chinese medicine with no effect on her ulcer. She also admitted to accompanying pain and obvious oedema of the lower extremities. The patient denied any tobacco or alcohol use or drug allergy. The physical exam was unremarkable except for an ulcer of 2.5 cm × 1.5 cm at the right foot lateral malleolus, with exudation and pus moss on or surrounding the wound; swelling and pain of the tissue were also observed. Laboratory reports including glucose 17.4 mmol/L, albumin 29.5 g/L, transaminase, bilirubin and creatinine were normal and triglyceride 3.47 mmol/L (other hospital), total cholesterol 9.58 mmol/L (other hospital), and multiple tumour markers were also normal. Her haemoglobin A1C was 14.6% (other hospital), indicating poor control of her glucose. The transcutaneous oxygen pressure (TcPO2) around the wound was 25–30 mmHg, suggesting that the oxygen and blood supply to the ulcer were strongly decreased. The ankle/brachial index (ABI) of the right lower limb was between 0.9–1.0, and the ABI of the left lower limb was between 1.0–1.3, within the normal range (0.9–1.3), suggesting that the leg blood supply was normal. The sensory threshold determination indicated that the patient had a foot touch pressure of 10 g nylon, multifaceted sensory loss and a serious deep sensory disturbance. Electromyography revealed that the multiple nerve sensory conduction and motor conduction velocity were significantly decreased. X-ray examination in another hospital suggested that there was no obvious abnormality in the right side of the right side of the ankle. However, the wound had obvious swelling, heat pain and inflammation. Therefore, we checked the magnetic resonance of the foot. Magnetic resonance imaging revealed right limb and right lateral malleolus injury; right ankle joint cavity effusion; and right lateral malleolus and foot root soft tissue oedema (for all of the abovementioned inspection reports, see Table 1). Patients with DFUs typically take a long time to recover. Although her liver and kidney function were normal, her glucose and lipid levels had not been well controlled for a long time, and her urine protein levels were elevated, which suggested early nephropathy. Electromyography suggested neuropathy. Although the ABI of the lower extremities was normal, the Tcpo2 was significantly reduced, suggesting that the local microcirculation was poor. According to the literature and clinical experience (1,2), traditional treatment such as glucose regulation, blood lipid control, debridement, and continuous negative pressure suction may be ineffective. Thus, we suggested to the patient that autologous BMMSC transplantation and APG may have some effect on wound healing on the basis of traditional treatment, and she agreed to undergo the autologous BMMSC transplantation and APG for DFU treatment. A similar treatment protocol had been approved by the First Affiliated Hospital of the Third Military Medical University Institutional Review Board, conformed to the standards of the Declaration of Helsinki and was registered with ClinicalTrials.gov, NCT 03248466 previously. The patient started the treatment with insulin to regulate her blood glucose (the final treatment scheme of insulin subcutaneous injection was 14 U of lispro before breakfast, 12 U of lispro before lunch, 10 U of lispro before supper, and 32 U of glargine at night); aspirin (100 mg once a day); alprostadil (10 µg once a day) to improve circulation; atorvastatin (20 mg once a night) to reduce cholesterol; and appropriate antibiotics (Ceftazidime, 2 g twice a day). After debridement and dressing, we treated the ulcer with continuous negative pressure suction for 2 weeks. The patient gained good blood glucose control (on the day of transplantation, her fasting blood glucose was 6.2 mmol/L, postprandial blood glucose was 6.8–8.4 mmol/L) and other parameters were improved (see Table 1). Additionally the wound was improved, only a little exudation and no pus or moss coverage were observed, and the swelling and pain of the surrounding tissue was reduced. On 2 August 2017, after foot debridement, the autologous BMMSCs (cell: 2×108/L) were subcutaneously injected around the wound. After injection, the APG (platelets: 1,165×109/L) was covered with wound dressing (see Figure 1A,B). The dressing was changed once every 5–7 days (see Figure 1B,C). After 14 days, the wound was significantly improved. Because there was no obvious ischaemia and the granulation of the base was tender and rosy, to accelerate healing, we sutured the wound (see Figure 1D). After 21 days and after 30 days, the wound was healed (see Figure 1E). The patient had no specific discomfort or adverse reaction at the end of the observation period.
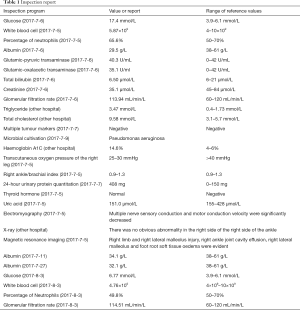
Full table
Discussion
The DFU is the most serious complication of diabetes. Diabetic patients are 40 times more likely to develop severe ischaemic disease of the lower extremity and undergo consequent amputations than non-diabetic patients. Approximately 85% of non-traumatic amputations are caused by diabetic foot disease, and 15% of diabetic patients experience at least one foot ulcer in their lives (2). Long-term hyperglycaemia, hypertension, and hyperlipidaemia lead to oxidative stress, and there is a lack of a blood and oxygen supply to the foot, which makes diabetic foot wounds difficult to heal and ultimately leads to amputation or death. Traditional therapies such as drugs, hyperbaric oxygen therapy, laser therapy and ultrasonic knife debridement are not effective (2). Therefore, on the basis of traditional therapy, the proper local management of foot ulcers has great significance towards improving the DFU outcome.
Wound healing is a complex process, involving coagulation, inflammation, cell migration, cell proliferation, and remodelling phases. DFU healing is an important problem; it consists of wound formation, peripheral vascular disease, neuropathy and infection (3). Researchers have suggested that the main reason for impaired DFU healing lies in the infection and insufficient blood and oxygen supply. Actually, proper wound management technologies, such as debridement, decompression, anti-infection treatment, hyperbaric oxygen therapy, revascularization and local dressing are needed; however, these measurements are not effective, and the amputation rate for diabetic patients remains higher than that of non-diabetic patients (4). Therefore, developing new measurements for DFU treatment to resist infection and improve the local blood and oxygen supply is essential.
In this case, the patient with the DFU did not recover over a long period of time in another hospital, and after she received the traditional treatment (insulin, aspirin, atorvastatin, antibiotics and negative pressure therapy) in our hospital, the ulcer was still poor healing, and some useful treatments needed to be applied to improve wound healing. Researchers have suggested that the local injection of autologous BMMSCs in the DFU and dressing coverage with an APG can improve the local blood oxygen partial pressure, accelerate wound healing, shorten the healing and hospitalization times, and avoid late amputation; however, no one has combined these two methods for DFU treatment. There have also been reports that BMMSCs and the APG can mediate local wound immune function, improve wound infection and promote wound healing. BMMSCs are able to secrete a variety of growth factors to improve the microcirculation of the DFU. BMMSCs can stimulate cell proliferation and differentiation and promote DFU repair through processes that involve growth factors, matrix remodelling, immune regulation and anti-inflammatory activities (5-7). The miRNAs in BMMSCs can induce the secretion of many cytokines and regulate many signalling pathways, which exert effects on inflammation, cell proliferation and maturation and lead to DFU healing (2). The in vitro amplification of BMMSCs in our research may provide more cells and cytokines, which are helpful to heal the wound. As a kind of biological material, the APG has played an important role in the treatment of diabetic foot disease; it contains a variety of growth factors, including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), connective tissue growth factor (CTGF), and TGF-β. Many studies have shown that APG can promote endothelial cell growth, improve local microcirculation, promote the migration of fibroblasts, promote the formation of the skin, regulate immune cell function, provide an antibacterial effect, and improve the local inflammatory reaction (8-10). These may be important mechanisms of autologous BMMSCs combined with APG in improving the clinical outcome of the DFU.
In conclusion, the results of this study are particularly promising in the field of tissue regeneration and suggest that on the basis of good traditional treatment and management that follow the current guideline, the combination of the APG and BMMSCs might provide a suitable and effective therapeutic tool for help DFU healing. Clearly, there is still much more work to be done to validate the bioengineered platform in clinical applications and to evaluate the actual role of the APG and BMMSCs in promoting DFU healing and tissue regeneration.
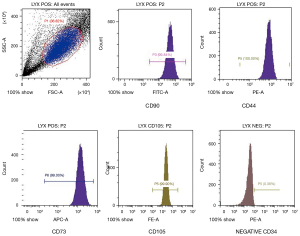
BMMSCs were obtained from a bone marrow puncture, and production, in vitro amplification and identification were performed by the biological treatment centre of our hospital (see Figure 2). Under strict aseptic conditions and local anaesthesia, bone marrow (30 mL) was obtained from the iliac crest of the patient. The obtained bone marrow was transferred to a clean room and subjected to Percoll (Pharmacia, 1.073 g/mL) density gradient centrifugation. Then, the mononuclear cell layer was harvested and cultured in flasks containing alpha-modified minimum essential medium (Invitrogen-Life Technologies Corp) supplemented with 10% autologous serum and incubated under normoxic conditions (21% O2 and 5% CO2). After proliferation in vitro, we took the 5th generation of cells for transplantation and FACS Calibur flow cytometry (Becton Dickinson and Company, San Jose, CA, USA). We examined surface molecule expression to identify the BMMSCs, and the following monoclonal antibodies were used: CD44, CD73, CD90, CD105 (BioLegend), and CD34 (Becton Dickinson, USA) (11).
Autologous platelet-rich plasma was obtained from the peripheral venous blood of the patient (approximately 200 mL, 200/g centrifugation for 10 min twice). The autologous platelet-rich plasma was mixed with 5,000 U of thrombin and 5 mL of 10% calcium chloride to generate the APG. The specific process of production has been previously described (8).
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (81370885) and the clinical trial project of the first affiliated Hospital of the Third Military Medical University in 2016 (SWH2016JSTSYB-11).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Zhang P, Lu J, Jing Y, et al. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med 2017;49:106-16. [Crossref] [PubMed]
- Wu Q, Bing C, Ziwen L. Mesenchymal stem cells as a prospective therapy for diabetic foot. Stem Cells Int 2016;2016. [Crossref] [PubMed]
- Bakker K, Apelqvist J, Lipsky BA, et al. International Working Group on the Diabetic Foot. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev 2016;32 Suppl 1:2-6. [Crossref] [PubMed]
- Frykberg RG, Zgonis T, Armstrong DG, et al. American College of Foot and Ankle Surgeons. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg 2006;45:S1-66. [Crossref] [PubMed]
- Kato J, Kamiya H, Himeno T, et al. Mesenchymal stem cells ameliorate impaired wound healing through enhancing keratinocyte functions in diabetic foot ulcerations on the plantar skin of rats. J Diabetes Complications 2014;28:588-95. [Crossref] [PubMed]
- Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3. [Crossref] [PubMed]
- Volarevic V, Arsenijevic N, Lukic ML, et al. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells 2011;29:5-10. [Crossref] [PubMed]
- Li L, Chen D, Wang C, et al. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair Regen 2015;23:495-505. [Crossref] [PubMed]
- Li L, Chen D, Wang C, et al. The Effect of Autologous Platelet-Rich Gel on the Dynamic Changes of the Matrix Metalloproteinase-2 and Tissue Inhibitor of Metalloproteinase-2 Expression in the Diabetic Chronic Refractory Cutaneous Ulcers. J Diabetes Res 2015;2015. [Crossref] [PubMed]
- Chen L, Wang C, Liu H, et al. Antibacterial effect of autologous platelet-rich gel derived from subjects with diabetic dermal ulcers in vitro. J Diabetes Res 2013;2013. [Crossref] [PubMed]
- Lu D, Chen B, Liang Z, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic crsitical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract 2011;92:26-36. [Crossref] [PubMed]


