Can we estimate transpulmonary pressure without an esophageal balloon?—yes
The lack of scientific consensus concerning positive end expiratory pressure (PEEP) setting is visualized by the numerous methods proposed for identifying optimal PEEP, such as decremental PEEP trial (1), best dynamic compliance trial (2) and center of ventilation (COV), regional ventilation delay (RVD index), global inhomogeneity (GI index), and intratidal gas distribution by electric impedance tomography (3). Selection of PEEP and tidal volume from airway pressure and total respiratory system compliance may be suboptimal, and lead to too high PEEP levels in some patients, such as patients with direct, pulmonary ARDS while in a patient with extrapulmonary acute respiratory distress syndrome (ARDS), where the chest wall influences respiratory mechanics, set PEEP will be to low (4). It is therefore obvious that lung and chest wall mechanics should be separated and transpulmonary pressure should be determined to provide a rational basis for selection of PEEP and tidal volume (5-10). However, the standard method to determine lung and chest wall mechanics using esophageal pressure measurements is technically complicated and difficult to interpret (8,11-13). This has led to a slow clinical introduction (8). We have developed a new, simple method based on a PEEP step maneuver to determine lung elastance and transpulmonary pressure (14-16).
Physiological background
Tidal esophageal pressure variations or changes in absolute esophageal pressure?
Esophageal pressure measurements have up to now been the only way of separating lung and chest wall mechanics, but there is no consensus on how to interpret absolute esophageal pressure. Thus, absolute esophageal pressure (PES) at FRC is accepted by some researchers as directly representative of absolute pleural pressure, in spite of the fact that PES is positive at FRC (11,13,17-22), while text books on respiratory physiology and several studies show that pleural pressure quite contrary, is negative, approximately 5–10 cmH2O (23-32). Such fundamental differences in the view on absolute esophageal pressure have made us refrain from using absolute esophageal pressure for the analysis of the elastic properties of the lung. Instead only tidal variations in esophageal pressure (∆PES), shown to have a good correlation with tidal pleural pressure variations (27,33), are used for calculation of chest wall and lung elastance and changes in end-expiratory transpulmonary and pleural pressure above FRC/ZEEP (for details on calculations, see Appendix).
Tidal and PEEP inflation
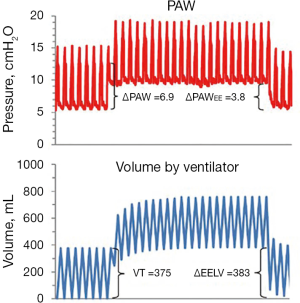
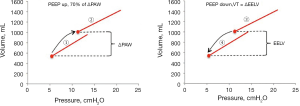
There are differences in the mechanic behaviors of the lung and chest wall when the lungs are inflated by a tidal volume compared to when they are inflated with an increase in PEEP. An increase in PEEP leads to a multi-breath inflation of the lung first described by Katz and coworkers in 1981 (34). They found that only about 70% of the total change in end-expiratory lung volume occurred during the first breath after PEEP. This was confirmed in a study on lung healthy patients (15). During several breaths after the initial breath there is a continuous increase in end-expiratory lung volume despite a constant end-expiratory airway pressure (PEEP). This is shown in a recording from a lung healthy patient (15) (Figure 1). In this patient the respiratory system elastance calculated as the increase in airway pressure divided by tidal volume was much higher than the change in PEEP divided by the increase in end-expiratory lung volume (6.9/0.375=18.4 cmH2O/L compared to 3.8/0.383=9.9 cmH2O/L).
The determinants of the change in end-expiratory lung volume following a PEEP increase
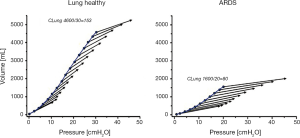
The difference in tidal and end-expiratory respiratory system elastance is observed both in lung healthy and ARDS patients (Figure 2). The form of the line connecting the end-expiratory airway pressure/volume (P/V) points is not a random phenomenon. It is obvious that it does not follow the respiratory system P/V-curves, which indicates that the increase in lung volume does not depend on respiratory system elastance. What factors do determine the change in lung volume after an increase of PEEP?
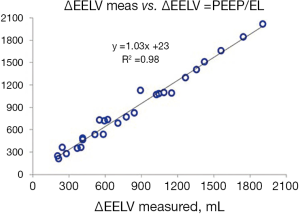
In a porcine study and a study on patients with acute respiratory failure it was shown that the change in lung volume after an increase of PEEP is dependent on the size of the PEEP-change and the elastance of the lung (14,16,36). Elastance of the lung was calculated using tidal changes in airway and esophageal pressures in these studies. The finding that the change in lung volume was dependent on lung elastance was further confirmed by analysis of data in three published studies. In two studies PEEP steps of 0-5-0-10-0-15 were performed on patients with healthy lungs, moderate and severe ARDS (37) and in patients with pulmonary and extrapulmonary ARDS (4). The change in end-expiratory lung volume was measured during a prolonged expiration to zero in PEEP. In the study on mixed ARDS patients, PEEP steps of 5 cmH2O were performed (from 5 to 40 cmH2O and back again) and changes in end-expiratory lung volume was measured as the cumulative difference in inspiratory and expiratory tidal volume between two steady state PEEP levels. The measured change in end-expiratory lung volume was closely correlated to the change in PEEP divided by lung elastance (Figure 3).
Measurements on lung healthy patients in a recently published study confirmed that lung elastance calculated from tidal changes in airway and esophageal pressures and the size of the PEEP-change determines the total change in end-expiratory lung volume after an increase of PEEP (Figure 4). The increase in end-expiratory lung volume after the first breath following an increase of PEEP was on the other hand dependent on the size of the PEEP change and respiratory system elastance (36).
Regarding the change in end-expiratory lung volume following an increase of PEEP we can so far conclude that:
- PEEP needs to be increased less than the airway driving pressure to induce an inflation of the lungs of the same size as the tidal volume.
- The change in EELV is determined by the change in PEEP divided by the elastance of the lung.
As a consequence, lung elastance can be determined by a PEEP step maneuver if the change in lung volume (∆EELV) is measured as:
EL = ∆PEEP/∆EELV
This may seem as a surprise, as a part of ∆EELV, the difference between ∆EELV and the “minimally predicted volume”, calculated as respiratory system compliance times ∆PEEP, has been claimed to be a measure of recruited volume (RecV) (39). However, it was later shown that RecV was twice as high in lung healthy patients with almost no alveolar collapse, as in ARDS patients with substantial collapse (35), which indicated that recruited volume mainly is a measure of a slow fraction of inflation of already aerated lung tissue, and not recruitment of collapsed alveoli.
The increase in end-expiratory transpulmonary pressure following a PEEP increase
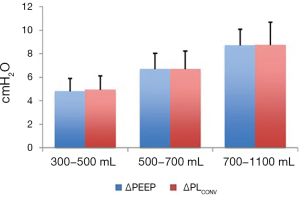
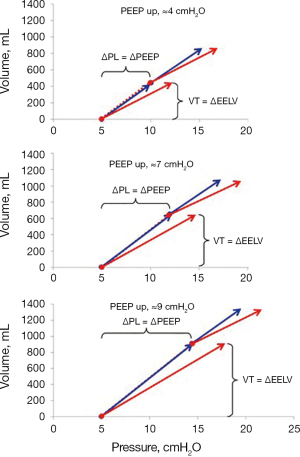
In an elastic structure such as the lung, the transpulmonary pressure will increase in relation to the inflated volume and the elastic properties of the lung, V × EL = PL. The mode of inflation, tidal or PEEP inflation, is irrelevant and as a consequence, the static steady state transpulmonary pressure at a certain lung volume is the same irrespective of if this volume has been reached by a tidal or a PEEP-induced inflation. Thus, in the same way as the transpulmonary driving pressure is calculated as the tidal volume times lung elastance, the increase in end-expiratory transpulmonary pressure in response to PEEP inflation can be calculated as the increase in end-expiratory lung volume times lung elastance. Such calculations were applied in a study on lung healthy patients where lung elastance was calculated using tidal changes in airway and esophageal pressures. Three different sizes of PEEP-changes were performed (4.8, 6.9 and 9.2 cmH2O) and the corresponding increase in end-expiratory transpulmonary pressure was 5.0, 7.0 and 9.4 cmH2O (15). At each of these PEEP steps a tidal volume was set to be equal to the change in end-expiratory lung volume and the transpulmonary driving pressure, of these tidal volumes were equal to the change in PEEP (Figure 5). In the studies by Pelosi et al., Gattinoni et al. and Garnero et al. described above, where PEEP steps of 5 cmH2O up to a level of 40 (!) cmH2O were used in patient with lung elastance ranging from 10 (lung healthy) to 25 cmH2O/L (severe ARDS), end-expiratory transpulmonary pressure increased 5.1 cmH2O when PEEP was increased by 5 cmH2O.
Since the transpulmonary pressure increases as much as the PEEP-increase, the lung P/V curve of a PEEP trial will coincide with the end-expiratory airway P/V curve, as shown in a PEEP trial in patients with acute lung injury (14) (Figure 6).
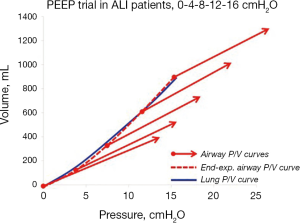
Thus, by performing a PEEP step maneuvers the lung pressure-volume curve can be plotted without using esophageal pressure measurements.
The role of the chest wall during tidal volume and PEEP inflation
The chest wall will contribute to the mechanic behavior of the respiratory system in different ways during tidal volume and PEEP inflation. The end-expiratory transpulmonary pressure increases as much as PEEP, which indicates that the calculated end-expiratory pleural pressure does not increase. This is not really a surprising finding as the recoil of the lung is balanced by the expanding force of the rib cage, creating a negative pleural pressure of −5 to −10 cmH2O at end-expiration at functional residual capacity (23-28,40). The chest wall is striving outwards until it reaches a resting volume at 70–80% of total lung capacity that is around 3 L above FRC (25,41-43). In the volume range between FRC and the chest wall resting volume, end-expiratory pleural pressure is negative irrespective of the pressure inside the lung. During mechanical ventilation, the thoracic cage, the diaphragm, the abdomen and its wall interact to form a “chest wall complex”. During inflation the diameter of the caudal rim of the rib cage is increased, the diaphragm is tensed and the diaphragmatic dome is displaced in caudal direction. The driving pressure of the chest wall complex during tidal inspiration is mainly related to the force needed to displace the abdominal weight by the push from the lung when inflated (33,44,45) and not to overcome any elastic recoiling force of the chest wall. As a consequence, the chest wall complex reacts to lung inflation as a weight (the abdomen) that is displaced, rather than an elastic entity that is inflated (44,46). The elastance of the chest wall during tidal inflation is therefore unchanged even when PEEP is increased (47). During tidal expiration the initial flow is high because of the recoil of the lung, but as expiration proceeds, the rib cage counteracts the recoil of the lung and will cause a termination of expiratory flow (48). Thus, the effect of these opposing forces keeps the end-expiratory pleural pressure negative and the chest wall complex off-loaded from the lung. When lung volume is increased by application of PEEP, the lung is inflated with a constant pressure until the new pressure volume equilibrium is reached. The force to inflate the lung and displace the weight of the abdomen (44,49,50) is exerted during the inspirations of the breaths involved in establishing this new equilibrium. The increase in lung volume is caused by the rib cage spring out force braking and eventually stopping the expirations and thereby detaining a part of the expiration. As a consequence, the end-expiratory pleural pressure remains negative at the new P/V equilibrium as long as the end-expiratory exterior lung volume (irrespective of pressure inside the lung) is below the resting volume of the rib cage, schematically shown in Figure 7.
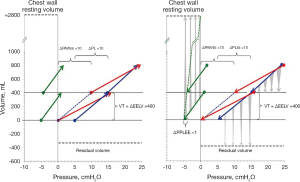
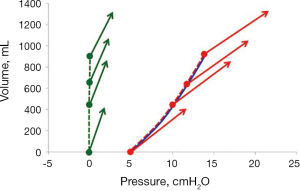
The difference between tidal chest wall elastance and end-expiratory chest wall elastance was confirmed in the study on lung healthy patients during anesthesia (15) (Figure 8).
The PEEP step measurement procedure
Lung elastance and consequently transpulmonary pressure can be determined without esophageal pressure measurements by a PEEP step procedure as described recently (15) (Figure 9). For optimal results a number of important requirements must be met:
- Volume control ventilation mode with at least 10% end-inspiratory pause;
- The change in lung volume (∆EELV) preferably determined by “The modified cumulative expiratory tidal volume difference from baseline” method (15,54);
- Tidal volume should be set close to the PEEP step induced change in ∆EELV;
- No spontaneous breathing;
- No intrinsic PEEP present.
When this procedure was tested on lung healthy patients during anesthesia in a recent study PEEP steps of ≈5, ≈7, and ≈9 cmH2O were applied (15). Lung elastance calculated from tidal changes in airway and esophageal pressures was almost equal to end-expiratory airway elastance (Figure 10).
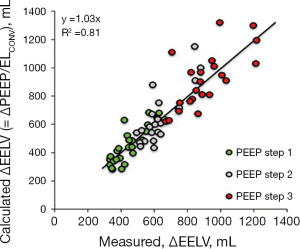
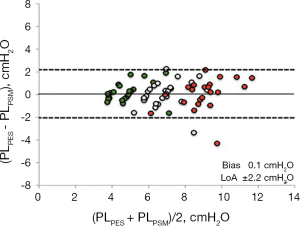
By performing a PEEP step maneuver lung elastance may be calculated as ∆PEEP/∆EELV and as a consequence, transpulmonary pressure can be determined as (∆PEEP/∆EELV) × VT. A Bland & Altman analysis (55) of the transpulmonary driving pressure derived from esophageal pressure (∆PLPES) measurements and derived from a PEEP step procedure (∆PLPSM) in lung healthy patients showed a bias of 0.1 cmH2O and limits of agreement of −2.2 to 2.4 cmH2O (Figure 11).
It is worth commenting the measurement precision in the PEEP step method. The PEEP step procedure is dependent on measurement of the difference in end-expiratory airway pressure between two PEEP levels and determination of the change in end-expiratory lung volume (∆EELV). The PEEP level is maintained by the ventilator with extreme precision and consequently, ∆PEEP is a very reliable measurement. It has been argued that ∆EELV should be measured as the difference between EELV measured at the high and low PEEP level by the nitrogen washin/washout method (56), but this method has a variability of ±10%, which in a patient with in a case with an EELV of 1,500 mL at the low PEEP and 1,800 mL at the high PEEP level, i.e., a ∆EELV of 300 mL, the EELV at the low PEEP can be between 1,350 and 1,650 mL. EELV at the high PEEP level can be between 1,620 and 1,980 mL. As a consequence ∆EELV can be between 630 and –30 mL, which is an unacceptable span. Consequently, ∆EELV should instead be determined by the cumulative tidal volume difference method (54), modified as described by Persson et al. 2018 (15), which in principle is a direct measurement of ∆EELV as inspiratory tidal volume is maintained constant even when PEEP is changed. Thus, ∆EELV measured by the modified cumulative difference in expiratory tidal volume of the breaths involved in establishing a new PEEP/EELV equilibrium, compared to baseline expiratory tidal volume, has a variability of below ±5%, i.e., a true ∆EELV of 300 mL can be measured as 285–315 mL.
Estimation of the lung P/V curve
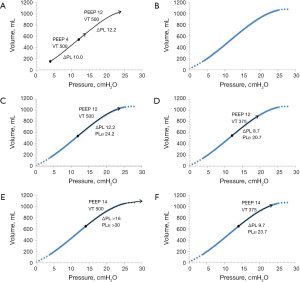
Tidal chest wall elastance at baseline PEEP, can be determined as the difference in respiratory system elastance (∆PAW/VT) and lung elastance (∆PEEP/∆EELV). As the tidal variations in esophageal pressure is related to the force needed to displace the weight of the abdomen (44,49,50), not the inflation of an elastic structure, chest wall elastance does not change when increasing PEEP, as the weight remains constant. Consequently, tidal chest wall elastance is almost constant during increasing PEEP levels (4,14,16,18,37,38,47,57). As a consequence, the end-inspiratory transpulmonary pressure at the high PEEP level of the procedure can be estimated as end-inspiratory airway plateau pressure minus tidal chest wall elastance times the tidal volume. Applying this on the PEEP step procedure makes it possible to obtain an estimated lung P/V curve from end-expiration at baseline PEEP to end-inspiration at the high PEEP. This constitutes a substantial part of the clinically useful lung P/V curve, when applying protective ventilation. The tidal transpulmonary P/V curve is positioned on this lung P/V curve. In a clinical situation, when deficient oxygenation requires changes in PEEP and/or tidal volume, the consequences of such on both transpulmonary driving pressure and end-tidal transpulmonary plateau pressure can be predicted with a fair precision (Figure 12) and the estimated lung P/V curve can be used as a clinical decision support.
Summary of the PEEP step method
- The change in EELV after an increase of PEEP is dependent of the size of the PEEP change and the elastance of the lung.
- The change in end-expiratory transpulmonary pressure is equal to the change in PEEP because of the chest wall spring out force.
- Lung elastance may as a consequence be calculated by a PEEP step maneuver where the change in EELV is measured and then used for calculations of transpulmonary driving pressure.
- There is good correlation between calculations of transpulmonary driving pressure with the PEEP step method and the conventional method using esophageal pressure measurements.
- The PEEP step method is based on calculations from changes in PEEP and differences between inspiratory and expiratory tidal volumes, which all are measured with high precision.
- With a PEEP step maneuver it is possible to plot the lung P/V-curve which in turn may be used as a decision support when aiming for lung protective ventilation.
When choosing between the conventional esophageal pressure method and the PEEP step procedure, it seems likely that the PEEP step procedure will result in measurements with high precision. As it is a simple, non-invasive procedure, it would probably be preferable in many situations. The procedure can be repeated to keep close track on the evolution of lung mechanics. Also, the PEEP step procedure offers a possibility for decision support that should be valuable in a clinical setting when a protective ventilation strategy is implemented.
Appendix
Acknowledgements
None.
Footnote
Conflicts of Interest: S Lundin and O Stenqvist are shareholders in The Lung Barometry Sweden AB. P Persson has no conflicts of interest to declare.
References
- Girgis K, Hamed H, Khater Y, et al. A decremental PEEP trial identifies the PEEP level that maintains oxygenation after lung recruitment. Respir Care 2006;51:1132-9. [PubMed]
- Suarez-Sipmann F, Bohm SH, Tusman G, et al. Use of dynamic compliance for open lung positive end-expiratory pressure titration in an experimental study. Crit Care Med 2007;35:214-21. [Crossref] [PubMed]
- Blankman P, Hasan D, Erik G, et al. Detection of 'best' positive end-expiratory pressure derived from electrical impedance tomography parameters during a decremental positive end-expiratory pressure trial. Crit Care 2014;18:R95. [Crossref] [PubMed]
- Gattinoni L, Pelosi P, Suter PM, et al. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med 1998;158:3-11. [Crossref] [PubMed]
- Cortes GA, Marini JJ. Two steps forward in bedside monitoring of lung mechanics: transpulmonary pressure and lung volume. Crit Care 2013;17:219. [Crossref] [PubMed]
- Plataki M, Hubmayr RD. Should mechanical ventilation be guided by esophageal pressure measurements? Curr Opin Crit Care 2011;17:275-80. [Crossref] [PubMed]
- Brochard L. Measurement of esophageal pressure at bedside: pros and cons. Curr Opin Crit Care 2014;20:39-46. [Crossref] [PubMed]
- Akoumianaki E, Maggiore SM, Valenza F, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014;189:520-31. [Crossref] [PubMed]
- Chiumello D. Transpulmonary pressure: a more pathophysiological open lung approach?*. Crit Care Med 2012;40:2249-50. [Crossref] [PubMed]
- Gattinoni L, Carlesso E, Brazzi L, et al. Positive end-expiratory pressure. Curr Opin Crit Care 2010;16:39-44. [Crossref] [PubMed]
- Gulati G, Novero A, Loring SH, et al. Pleural pressure and optimal positive end-expiratory pressure based on esophageal pressure versus chest wall elastance: incompatible results*. Crit Care Med 2013;41:1951-7. [Crossref] [PubMed]
- Gattinoni L, Cressoni M, Chiumello D, et al. Transpulmonary Pressure Meaning: Babel or Conceptual Evolution? Am J Respir Crit Care Med 2017;195:1404-5. [Crossref] [PubMed]
- Loring SH, Topulos GP, Hubmayr RD. Transpulmonary Pressure: The Importance of Precise Definitions and Limiting Assumptions. Am J Respir Crit Care Med 2016;194:1452-7. [Crossref] [PubMed]
- Lundin S, Grivans C, Stenqvist O. Transpulmonary pressure and lung elastance can be estimated by a PEEP-step manoeuvre. Acta Anaesthesiol Scand 2015;59:185-96. [Crossref] [PubMed]
- Persson P, Stenqvist O, Lundin S. Evaluation of lung and chest wall mechanics during anaesthesia using the PEEP-step method. Br J Anaesth 2018;120:860-7. [Crossref] [PubMed]
- Stenqvist O, Grivans C, Andersson B, et al. Lung elastance and transpulmonary pressure can be determined without using oesophageal pressure measurements. Acta Anaesthesiol Scand 2012;56:738-47. [Crossref] [PubMed]
- Loring SH, O'Donnell CR, Behazin N, et al. Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol 2010;108:515-22. [Crossref] [PubMed]
- Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008;359:2095-104. [Crossref] [PubMed]
- Talmor D, Sarge T, O'Donnell CR, et al. Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med 2006;34:1389-94. [Crossref] [PubMed]
- Talmor DS, Fessler HE. Are esophageal pressure measurements important in clinical decision-making in mechanically ventilated patients? Respir Care 2010;55:162-72; discussion 172-4. [PubMed]
- Talmor DS, Loring SH. Esophageal pressures in acute respiratory distress syndrome: how should we interpret and use them? Crit Care Med 2013;41. [Crossref] [PubMed]
- Chiumello D, Cressoni M, Colombo A, et al. The assessment of transpulmonary pressure in mechanically ventilated ARDS patients. Intensive Care Med 2014;40:1670-8. [Crossref] [PubMed]
- Donders FC. Beiträgezum Mechanismus der Respiration und Circulation in gesunden und kranken Zustände. Z Ration Med 1853;3:287-319.
- Ferris BG Jr, Mead J, Frank N. Effect of body position on esophageal pressure and measurement of pulmonary compliance. J Appl Physiol 1959;14:521-4. [Crossref]
- Finucane KE, Colebatch HJ. Elastic behavior of the lung in patients with airway obstruction. J Appl Physiol 1969;26:330-8. [Crossref] [PubMed]
- Mead J, Gaensler EA. Esophageal and pleural pressures in man, upright and supine. J Appl Physiol 1959;14:81-3. [Crossref] [PubMed]
- Pelosi P, Goldner M, McKibben A, et al. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 2001;164:122-30. [Crossref] [PubMed]
- Wiener-Kronish JP, Gropper MA, Lai-Fook SJ. Pleural liquid pressure in dogs measured using a rib capsule. J Appl Physiol (1985) 1985;59:597-602. [Crossref] [PubMed]
- Hoffman EA, Lai-Fook SJ, Wei J, et al. Regional pleural surface expansile forces in intact dogs by wick catheters. J Appl Physiol Respir Environ Exerc Physiol 1983;55:1523-9. [PubMed]
- Nunn JF. Nunn’s applied respiratory physiology. Oxford: Butterworth-Heinemann Ltd; 1993.
- West JB. Ventilation, blood flow and gas exchange. 3 ed. Oxford: Blackwell; 1977.
- Agostoni E, Mead J. Statics of the respiratory system. In: Fenn WO, Rahn H. editors. Handbook of Physiology. Washington: American Physiological Society, 1973:387-409.
- Gattinoni L, Chiumello D, Carlesso E, et al. Bench-to-bedside review: chest wall elastance in acute lung injury/acute respiratory distress syndrome patients. Crit Care 2004;8:350-5. [Crossref] [PubMed]
- Katz JA, Ozanne GM, Zinn SE, et al. Time course and mechanisms of lung-volume increase with PEEP in acute pulmonary failure. Anesthesiology 1981;54:9-16. [Crossref] [PubMed]
- Stahl CA, Moller K, Steinmann D, et al. Determination of 'recruited volume' following a PEEP step is not a measure of lung recruitability. Acta Anaesthesiol Scand 2015;59:35-46. [Crossref] [PubMed]
- Persson P, Stenqvist O, Lundin S. Evaluation of lung and chest wall mechanics during anaesthesia using the PEEP-step method. Br J Anaesth 2018;120:860-7. [Crossref] [PubMed]
- Pelosi P, Cereda M, Foti G, et al. Alterations of lung and chest wall mechanics in patients with acute lung injury: effects of positive end-expiratory pressure. Am J Respir Crit Care Med 1995;152:531-7. [Crossref] [PubMed]
- Garnero A, Tuxen D, Ducros L, et al. Non-invasive assessment of lung elastance in patients with acute respiratory distress syndrome. Minerva Anestesiol 2015;81:1096-104. [PubMed]
- Dellamonica J, Lerolle N, Sargentini C, et al. PEEP-induced changes in lung volume in acute respiratory distress syndrome. Two methods to estimate alveolar recruitment. Intensive Care Med 2011;37:1595-604. [Crossref] [PubMed]
- Hoffman EA, Behrenbeck T, Chevalier PA, et al. Estimation of regional pleural surface expansile forces in intact dogs. J Appl Physiol Respir Environ Exerc Physiol 1983;55:935-48. [PubMed]
- Christie RV. The Elastic Properties of the Emphysematous Lung and Their Clinical Significance. J Clin Invest 1934;13:295-321. [Crossref] [PubMed]
- Hurtado A, Kaltreider NL, Fray WW, et al. Studies of Total Pulmonary Capacity and Its Sub-Divisions. Vi. Observations on Cases of Obstructive Pulmonary Emphysema. J Clin Invest 1934;13:1027-51. [Crossref] [PubMed]
- Sciurba FC, Rogers RM, Keenan RJ, et al. Improvement in pulmonary function and elastic recoil after lung-reduction surgery for diffuse emphysema. N Engl J Med 1996;334:1095-9. [Crossref] [PubMed]
- Hedenstierna G. Esophageal pressure: benefit and limitations. Minerva Anestesiol 2012;78:959-66. [PubMed]
- El-Dash SA, Borges JB, Costa EL, et al. There is no cephalocaudal gradient of computed tomography densities or lung behavior in supine patients with acute respiratory distress syndrome. Acta Anaesthesiol Scand 2016;60:767-79. [Crossref] [PubMed]
- Agostoni E. Mechanics of the pleural space. In: Maklem I, Mead J. editors. Handbook of Physiology. Bethesda, Maryland: Am Physiol Soc., 1986:531-9.
- Fumagalli J, Berra L, Zhang C, et al. Transpulmonary Pressure Describes Lung Morphology During Decremental Positive End-Expiratory Pressure Trials in Obesity. Crit Care Med 2017;45:1374-81. [Crossref] [PubMed]
- Chelucci GL, Brunet F, Dall'Ava-Santucci J, et al. A single-compartment model cannot describe passive expiration in intubated, paralysed humans. Eur Respir J 1991;4:458-64. [PubMed]
- Agostini E, Hyatt R. The respiratory system. Mechanics of breathing. Handbook of Physiology. Bethesda: American Physiological Society; 1986. p. 113-30.
- Rahn H, Otis AB, Chadwick LE, et al. The pressure-volume diagram of the thorax and lung. Am J Physiol 1946;146:161-78. [Crossref] [PubMed]
- Nunn J. Elastic forces and lung volumes. In: Nunn JF. editor. Nunn’s applied respiratory physiology. 4 ed. Oxford: Butterworth-Heinemann, 1995:36-52.
- West JB. Mechanics of breathing. In: West JB. editor. Respiratory physiology. 3rd ed. Baltimore, Hong Kong, London, Sydney: Williams & Wilkins, 1985:85-111.
- Persson P, Lundin S, Stenqvist O. Transpulmonary and pleural pressure in a respiratory system model with an elastic recoiling lung and an expanding chest wall. Intensive Care Med Exp 2016;4:26. [Crossref] [PubMed]
- Grivans C, Lundin S, Stenqvist O, et al. Positive end-expiratory pressure-induced changes in end-expiratory lung volume measured by spirometry and electric impedance tomography. Acta Anaesthesiol Scand 2011;55:1068-77. [Crossref] [PubMed]
- Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet 1995;346:1085-7. [Crossref] [PubMed]
- Olegård C, Sondergaard S, Houltz E, et al. Estimation of functional residual capacity at the bedside using standard monitoring equipment: a modified nitrogen washout/washin technique requiring a small change of the inspired oxygen fraction. Anesth Analg 2005;101:206-12. table of contents. [Crossref] [PubMed]
- Katz JA, Zinn SE, Ozanne GM, et al. Pulmonary, chest wall, and lung-thorax elastances in acute respiratory failure. Chest 1981;80:304-11. [Crossref] [PubMed]
- Staffieri F, Stripoli T, De Monte V, et al. Physiological effects of an open lung ventilatory strategy titrated on elastance-derived end-inspiratory transpulmonary pressure: study in a pig model*. Crit Care Med 2012;40:2124-31. [Crossref] [PubMed]