Molecular basis of cystic fibrosis: from bench to bedside
Introduction
Cystic fibrosis (CF), the most common life-threatening rare disease among Caucasians, is an autosomal recessive genetic disease occurring in approximately one in 3,000–4,000 live birth as based on neonatal screening (1). Although several organs are involved, manifestation of CF disease in the airway tract is the main cause of mortality and morbidity in these patients (2). From the first description of a disease of the exocrine pancreas associated with lung symptoms in 1938 (3), survival of CF patients increased to a median age of 40 years, thanks to antibiotic therapies and correcting the intestinal malabsorption (4,5). After the identification of defective CF transmembrane conductance regulator (CFTR) gene, in 1989 (6-8), therapeutic approaches on the management of symptoms had a turning point which opened many hopes towards CFTR gene-targeted strategies (9), a field of investigation full of promises in steady progress.
Role of CFTR protein
CF disease is due to the defect of the CFTR gene located on chromosome 7 (6). CFTR gene encodes a protein encompassing the cellular membrane with two membrane-spanning domains (MSD), each constituted by six alpha-helices, two cytoplasmic domains, each binding one ATP molecule, termed nucleotide binding domains (NBD), a regulatory (R) domain with several consensus sequences for phosphorylation by protein kinase A (PKA) and protein kinase C (PKC). CFTR protein belongs to the family of ATP-binding cassette (ABC) transmembrane proteins (10). It is a chloride ion transporter localized at the apical membrane of several polarized epithelia (11,12), although other small molecules seem to be transported by CFTR (13), including ATP (14-17). As a chloride transporter, CFTR plays a critical role in the hydration of the mucus at the surface of the airway tract (18). Moreover, it favors mucus tethering and detachment through alkalinization with bicarbonate (19,20). De-hydration of airway surface fluid is a critical feature in the onset of the neutrophil dominated inflammatory and infective milieu of CF airways, which begins in the early months of life (21). CFTR-mediated ion transport requires binding of ATP on NBFs and phosphorylation of the R domain by protein kinase A (22-25) and protein kinase C (26-28).
Lung disease in CF
Defective ion transport mediated by CFTR reduces airway surface liquid hydration, which impairs mucociliary clearance, one of the basic innate immune defense mechanism of the respiratory tract (18,21). CF lung disease is characterized by an exaggerated inflammatory response accompanied by a huge number of neutrophils in the lumen of bronchi (21). However, these neutrophils are unable to completely clear bacteria; thus, repeated infections, mainly by Haemophilus influenzae and Staphylococcus aureus, pave the way to a chronic settlement of Pseudomonas aeruginosa. In addition, neutrophils release proteases, mainly elastases, reactive oxygen species and neutrophil extracellular traps thus worsening respiratory function and progressive tissue destruction and ultimately leading to respiratory insufficiency, reduced quality and expectancy of life (21,29-34). Experiments performed in different model systems in vitro, ex vivo and in vivo animal models have not yet clarified whether recruitment of neutrophils in the bronchial lumen, precedes or follows bacterial infection (35-37). To combat respiratory insufficiency, CF patients are treated with antibiotics and anti-inflammatories and soon or later, they undergo lung transplantation, which provides a dramatic improvement in the quality of life and some extension of survival (38-40).
The CFTR gene mutations
The 250-kb gene, located in chromosome 7, is structured in 27 exons. An International Worldwide Consortium of laboratories of molecular genetics extensively analyzed sequence variants and to date, over 2,000 sequence variations have been reported, at least 200 of them being associated with the disease (see the Cystic Fibrosis Mutation Database of the Cystic Fibrosis Gene Analysis Consortium, www.genet.sickkids.on.ca/cftr/) (41,42). Deletion of the phenylalanine in position 508 of the polypeptide chain, known as Phe508del or F508del, is the most common CFTR mutation, affecting from 50% to 90% of the chromosomes of CF patients along different geographical areas (43). Besides F508del CFTR, most CF causing mutations are missense variants (42%), nonsense (10%), frameshift (15%), splicing (13%), in frame deletion/insertion (2%) and promoter (0.5%) mutations (42,43).
The molecular defects of CFTR protein
F508del CFTR mutation (8) leads to the synthesis of an immature, non-glycosylated protein unable to localize on the plasma membrane (44). In-depth studies on the consequences of the different mutations on CFTR protein have allowed to simplify functional defect mechanisms (45), now schematized into the six classes (2), as shown in Figure 1 and described as follows:
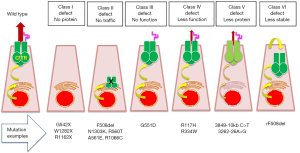
Class I—“No protein”
These mutations affect protein synthesis, due to stop-codon (nonsense) mutations in which the CFTR mRNA is degraded through a process termed nonsense-mediated decay. This class includes G542X mutation (common in Mediterranean coastal area), R1162X (common in North-eastern Italy and Catalonia), W1282X (affecting about 40% chromosomes in Ashkenazi Jews).
Class II—“No traffic”
These mutations affect CFTR protein processing, due to protein misfolding, which is recognized by the endoplasmic reticulum (ER) quality control machinery leading to protein degradation. This class includes the most common F508del mutation, N1303K, R560T, A561E and R1066C.
Class III—“No function”
These mutations, also termed as “gating defect”, affect the activation of ion transport function, although CFTR is correctly glycosylated and located at the plasma membrane. This class includes G551D mutation.
Class IV—“Less function”
These mutations reduce chloride ions transported through pore channel, due to mutations the arginine located in the MSDs, which are involved in the flow of chloride through the plasma membrane. This class includes R117H and R334W.
Class V—“Less protein”
These mutations significantly reduce the amount of wild-type CFTR protein at the plasma membrane, mainly due aberrant splicing of RNA, leading to a non-functional protein. This class includes 3849-10 kb C>T and 3262-26A>G.
Class VI—“Less stable”
These mutations affect the stability and/or anchoring of CFTR protein at the plasma membrane. This class includes F508del CFTR rescued (rF508del) by correctors.
Notably, many CFTR causing mutations are not classified in one of these six classes and in some cases, mutations present more than one class defect, e.g., F508del mutation has a processing defect (class II), a gating defect (class III) and a reduced stability at the plasma membrane after being rescued (class VI). Despite simplistic, this classification has focused research of novel drugs towards different protein defects thus allowing development of personalized medicine, i.e., specific treatments tailored on CF genotype (46).
High throughput screening (HTS) in search of new CFTR protein targeted molecules
In search of CFTR modulators, large scale chemical libraries comprising thousands of compounds were tested. Initial challenge was to set up simple and rapid technological tools to study the effect of each molecule on chloride channel activity. In this respect, three different HTS assays have been developed, as reviewed and depicted in Figure 2 (50). Starting from the SPQ molecule, whose emission intensity is modulated by intracellular collisional quenching, other halide-sensitive fluorescent probes have been developed, such as MQAE, a membrane permeable dye retained inside the cells by cleavage of acetyl ester residues (47,51-53). A second assay, based on membrane depolarization dependent on chloride channels activation under proper experimental conditions has been set up. In this assay, membrane depolarization can be detected by measuring variations of fluorescence of membrane-potential sensitive dyes, due to the quantum yield change upon different polarity of the cellular environment (54,55). We developed the membrane-potential sensitive probe bis-oxonol to detect CFTR correction after transferring with viral vectors the wild type CF gene in CF bronchial epithelial cells (48). This assay was then accomplished by an HTS of more than 100,000 compounds that lead to the discovery of the first two small molecules became drugs for CF patients: VX-770 and VX-809 (56-58). A third tool, based on dynamic quenching of a yellow-fluorescent protein (YFP) made sensitive to intracellular chloride-ion concentration was set up (59) and further improved by mutations that render YFP very sensitive to chloride ion (49,60). F508del CFTR correctors and G551D CFTR potentiators were discovered by this assay (61-64). Interestingly, also a potent CFTR-specific inhibitor (65), currently used to inhibit CFTR function in vitro assays, was discovered and proposed to target the hyper-secretory diarrhea mediated by hyper-functional CFTR protein, induced by cholera toxin (65).

The first molecules reaching the chemist’s bench
CFTR correctors are the molecules able to rescue the class II defective CFTR, e.g., F508del CFTR and CFTR potentiators those activating the chloride transport in Class III gating-defective CFTR, e.g., G551D (2). This terminology allows to define the effect of each molecule and recalls the experimental conditions utilized in the screening. As a matter of fact, HTS for discovery correctors is performed in F508del CFTR expressing cells, incubated for 24–48 hours with the testing molecules whereas HTS for potentiators is carried out in G551D CFTR expressing cells, acutely treated with such compounds (50). Firstly, CFTR modulators were identified by academic groups (61-65), however these molecules did not undergo a pharmaceutical development from preclinical to clinical trials. Importantly, the biotech company Vertex Pharmaceuticals published its first “CFTR corrector” VX-809 (Lumacaftor) (56,58) and its first “CFTR potentiator” VX-770 (Ivacaftor, trade name Kalydeco) (57), few years later.
These molecules underwent a quick drug development passing from in vitro assays (56-58) directly to clinical trials in CF patients. Food and Drug Administration approved VX-770 in 2012 and VX-809 in 2015, for the treatment of CF patients carrying specific CFTR mutations.
VX-770 has proven excellent efficacy in children over six years of age and adults with G551D mutation in at least one allele (66-68), as demonstrated by an average 10% increase of forced expiratory volume in 1 second (FEV1), decrease of pulmonary exacerbations, weight increase and normalization of sweat electrolytes (67), also patients with very low residual lung function (e.g., FEV1 <40%) (69,70) or carrying class III mutations other than G551D (71). Unfortunately, the advantages of this drug are limited to very few CF patients as G551D mutation is very rare (43,72).
On the contrary, treatment with VX-809 in F508del CFTR homozygous patients did not produce any improvement of FEV1 (73). These disappointed results led to development of VX-770 and VX-809 combined formulation, named Orkambi that was tested in CF patients homozygous for the F508del CFTR mutation, providing some benefits in lung function (74). These data were reproduced in a large international multicentric clinical trial showing a 2–3% increase of FEV1 in respect to placebo after 24 weeks of treatment with Orkambi (75), although benefits were less evident in compound heterozygous CF patients carrying F508del CFTR in one allele (76). Different investigations in vitro have been pursued in order to understand these clinical limitations. For instance, it has been found that VX-770 negatively interacts with the rescued F508del CFTR protein by VX-809, thus reducing plasma membrane stability (77,78). How this interaction translates in CF patients is presently debated (79,80).
Molecules for class I “No protein” defects
As mentioned above, class I mutations cause CFTR mRNA degradation through nonsense-mediated decay. Discovery of molecules able to read-through the premature stop codons for treating CF patients carrying class I mutations started after the observation that aminoglycoside antibiotics can correct this defect (81,82). In this respect, the aminoglycoside gentamycin, previously used for the treatment of bacterial infections (83-86) was investigated. In order to avoid toxicity of aminoglycosides, gentamycin was then replaced by the analogue ataluren, produced by PTC Therapeutics (87-89). Unfortunately, a long-term placebo-controlled double-blind phase 3 study showed no improvement in the primary endpoint FEV1 in CF patients, despite initial promising findings in several clinical trials (90). This led PTC Therapeutics discontinuing the development of ataluren in CF, leaving wide open the need of compounds targeting class I mutations (91).
Molecules for class II “No traffic” defects
Several correctors to rescue the class II defective CFTR, e.g., F508del CFTR, have been discovered by different academic groups in the United States and Europe (64,92-115). However very few of them underwent pharmaceutical development. Thus, different pharmaceutical companies are investing their own resources in pre-clinical discovery of new correctors.
The encouraging advancements obtained with VX-809 prompted Vertex Pharmaceuticals to explore new correctors, such as VX-661 (Tezacaftor) in association with VX-770 (116-118). F508del CFTR homozygous patients, treated with this combination ameliorated lung function (116-118). In addition to VX-809 and VX-661, several other correctors discovered by Vertex Pharmaceuticals (VX-152, VX-440, VX-445, VX-659) and by other companies, such as Genzyme/Sanofi, Pfizer and Reata (FDL169, GLPG2222, PTI-428, PTI-801), entered in phase 1/2 clinical trials (https://www.cff.org/Research/Developing-New-Treatments/).
Molecules for class III “No function” defects
Treatment of F508del CFTR homozygous patients requires both correctors and potentiators to rescue the gating defect also present in F508del CFTR protein (119).
Approval of VX-770 for CF patients with G551D mutation (56), is one of the major breakthroughs for CF cure (66-68). Nevertheless, negative interactions between VX-770 and VX-809 (77,78) prompted academic groups to search novel potentiators that do not present these limitations (120). In parallel, other companies launched phase 2 and phase 1 clinical trials on new potentiators (https://www.cff.org/Research/Developing-New-Treatments/). Very interestingly, dual-acting compounds, i.e., corrector and potentiator activity, may be a very appealing therapeutic perspective for CF treatment (see below).
Molecules for class IV “Less function” defects
This mutated CFTR protein displays low ion conductance that could be repaired by increasing protein expression at the plasma membrane or potentiating its open state period. In this regard, clinical trials with VX-770 (Ivacaftor, Kalydeco) in CF patients carrying the R117H mutation showed some benefit in lung function of adults with stable disease (121). This evidence supports further testing of potentiators in patients with CFTR class IV mutations.
Molecules for class V “Less protein” defects
As detailed above, class V mutations reduce the expression of functional CFTR. As a consequence of abnormal splicing both aberrant and normal transcripts are produced. To repair this defect, increase CF gene transcription as well as CFTR correctors and potentiators could represent useful remedies (122).
Molecules for class VI “Less stable” defects
Less stable CFTR protein needs to strengthen its anchoring at the plasma membrane. Importantly, rescued F508del CFTR protein by correctors displays increased turnover due to its removal by the peripheral quality control machinery and Disabled-2 (Dab2)-dependent ubiquitination (123-126), further worsened by P. aeruginosa chronic infection that decreases, the expression of critical proteins, such as Na+/H+ exchanger regulatory factor 1 (NHERF1) (127-129). Therefore, treatment of F508del CFTR homozygous patients should be addressed not only with correctors and potentiators but also with compounds stabilizing the rescued CFTR, by targeting both the CFTR anchoring proteins and the peripheral quality control machinery.
Dual-acting CFTR corrector and potentiator compounds
Consensus was reached that multiple defects of F508del CFTR protein should be addressed by combination of correctors and potentiators [for review see (130)]. In order to avoid negative side effects due to multiple drug interactions, compounds able to act at the same time both as correctors and potentiators, i.e., dual-acting compounds, have been proposed (131,132). Several dual-acting compounds have been identified so far (99,103,104,133-135). In this regard, an interesting example has been given by 4,6,4'-trimethylangelicin (TMA) which besides correcting and potentiating CFTR activity displays anti-inflammatory properties (99,134). TMA exerts its dual action by interacting directly with the MSD1 on F508del CFTR protein (136).
The CFTR “amplifiers”
Beside the above mentioned molecular defects, F508del mutation produces a somewhat low amount of non-glycosylated immature Band B CFTR (44). Transcriptional inducers such as 4-phenylbutirrate were found to repair CFTR function by increasing band B CFTR protein that could escape at least in part the quality control systems (137,138). Therefore, forcing the production of band B CFTR protein in association with CFTR correctors could improve the overall efficacy of treatment (139). In this respect, new class of compounds called “CFTR amplifiers” seem to provide promising results in vitro (140). In particular, PTI-428 has been tested in a phase 1 clinical trial in CF patients under sponsorship of Proteostasis Therapeutics (https://www.cff.org/Research/Developing-New-Treatments/). An alternative approach is to inhibit the degradative pathway of CFTR mRNA intervening on the epigenetic down regulation of CFTR expression, e.g., by microRNA miR-145, which inhibits CFTR translation by degrading CFTR mRNA and blocking CFTR protein translation. MiR-145-specific cell permeable peptide-nucleic acid chimera relevantly increased CFTR protein (141).
Effects of repairing mutated CFTR on lung infection and inflammation
It has been suggested that repairing of the ion transport defect by CFTR correctors and potentiators can by itself solve CF chronic lung infection and inflammation. In vitro evidence supports this idea as VX-809 abolished the exaggerated inflammatory pathways in F508del CFTR bronchial epithelial cells (142,143). A different F508del CFTR corrector, miglustat, was also found to have anti-inflammatory effects in CF bronchial epithelial cells, although not directly related to correction of mutated CFTR (144). On the contrary, derivatives of the angular furocoumarin angelicin already proved as correctors of F508del CFTR protein (TMA analogues) showed that rescue and anti-inflammatory activity can coexist or be separated in the same molecule as a function of structural changes (145). These findings provide evidence that CFTR rescue per se is not enough to reduce excessive inflammation. Despite different results in vitro indicate that F508del CFTR rescue could per se repairs excessive inflammation (142,144), no evidence of reduced lung inflammation after VX-809 has been presented so far in CF patients. Moreover, CFTR restoration for all individuals with CF is challenging because approximately 2000 CFTR variants have been reported, most of them are rare (see the Cystic Fibrosis Mutation Database of the Cystic Fibrosis Gene Analysis Consortium, www.genet.sickkids.on.ca/cftr) and personalized medicine approaches based on each individual’s genetic profile may not be sufficiently efficacious in patients with irreversible lung damage. Thus, it appears that both combinations of novel CFTR potentiators and correctors as well as newer compounds for conventional therapies, such as inhaled antibiotics and anti-inflammatory agents, remain a cornerstone of treatment for CF lung disease [for review see (146-149)].
Conclusions and open issues
The ability to repair CF defect by using personalized medicine based on each patient’s genetic profile represents a new challenge for CF research community. Despite exciting advances, several issues still remain open:
- As not all CFTR gene mutations have been classified within the six classes and many defects lack repairing molecules, the right drug for each CF patient is not available yet;
- In order to increase the amount of rescued F508del CFTR, more effective correctors are still needed as well as clear-cut biomarkers to evaluate their efficacy;
- Considering that CF patients should be treated by combination of more drugs, the interaction between these drugs needs to be investigated in depth;
- Different therapeutic response along different patients is emerging, therefore, clinical efficacy of a specific treatment in every patient should be predicted by novel tools;
- Long-term safety of new drugs is still unknown;
- It is still a matter of debate whether rescuing CFTR defect avoids infection and exaggerated inflammation occurring in CF patients, thus newer compounds for conventional therapies, such as antibiotics and anti-inflammatories will likely remain a cornerstone of treatment for CF lung disease;
- Many questions are still open on the role of other genes, besides the CFTR one, in modulating pulmonary phenotype.
All this considered, a good therapeutic strategy should be based on more than one option.
Acknowledgements
Funding: This review was made possible by support of different research projects with grants from Telethon Foundation, CariVerona Foundation and Italian Cystic Fibrosis Research Foundation (FFC # 4/2004, 4/2005, 12/2008, 8/2010, 16/2010, 17/2010, 1/2011, 5/2011, 1/2012, 14/2012, 1/2013, 8/2014, 17/2014, 9/2015, 22/2015, 1/2016, 3/2016).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Farrell PM, Rosenstein BJ, White TB, et al. Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr 2008;153:S4-14. [Crossref] [PubMed]
- Elborn JS. Cystic fibrosis. Lancet 2016;388:2519-31. [Crossref] [PubMed]
- Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med 2006;173:475-82. [Crossref] [PubMed]
- Chmiel JF, Konstan MW, Elborn JS. Antibiotic and anti-inflammatory therapies for cystic fibrosis. Cold Spring Harb Perspect Med 2013;3. [Crossref] [PubMed]
- Cohen-Cymberknoh M, Shoseyov D, Kerem E. Managing cystic fibrosis. Strategies to increase life expectancy and improve quality of life. Am J respir Crit Care Med 2011;183:1463. [Crossref] [PubMed]
- Rommens JM, Iannuzzi MC, Kerem B, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 1989;245:1059-65. [Crossref] [PubMed]
- Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066-73. [Crossref] [PubMed]
- Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis. Science 1989;245:1073-80. [Crossref] [PubMed]
- Bell SC, De Boeck K, Amaral MD. New pharmacological approaches for cystic fibrosis: promises, progress, pitfalls. Pharmacol Ther 2015;145:19-34. [Crossref] [PubMed]
- Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol 1992;8:67-113. [Crossref] [PubMed]
- Trezise AE, Buchwald M. In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature 1991;353:434-7. [Crossref] [PubMed]
- Denning GM, Ostedgaard LS, Cheng SH, et al. Localization of cystic fibrosis transmembrane conductance regulator in chloride secretory epithelia. J Clin Invest 1992;89:339-49. [Crossref] [PubMed]
- Hasegawa H, Skach W, Baker O, et al. A multifunctional aqueous channel formed by CFTR. Science 1992;258:1477-9. [Crossref] [PubMed]
- Reisin IL, Prat AG, Abraham EH, et al. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J Biol Chem 1994;269:20584-91. [PubMed]
- Prat AG, Reisin IL, Ausiello DA, et al. Cellular ATP release by the cystic fibrosis transmembrane conductance regulator. Am J Physiol 1996;270:C538-45. [Crossref] [PubMed]
- Abraham EH, Okunieff P, Scala S, et al. Cystic fibrosis transmembrane conductance regulator and adenosine triphosphate. Science 1997;275:1324-6. [Crossref] [PubMed]
- Cantiello HF. Nucleotide transport through the cystic fibrosis transmembrane conductance regulator. Biosci Rep 1997;17:147-71. [Crossref] [PubMed]
- Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med 2007;261:5-16. [Crossref] [PubMed]
- Shah VS, Meyerholz DK, Tang XX, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 2016;351:503-7. [Crossref] [PubMed]
- Tang XX, Ostedgaard LS, Hoegger MJ, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest 2016;126:879-91. [Crossref] [PubMed]
- Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med 2015;372:1574-5. [Crossref] [PubMed]
- Rich DP, Anderson MP, Gregory RJ, et al. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature 1990;347:358-63. [Crossref] [PubMed]
- Egan M, Flotte T, Afione S, et al. Defective regulation of outwardly rectifying Cl- channels by protein kinase A corrected by insertion of CFTR. Nature 1992;358:581-4. [Crossref] [PubMed]
- Quinton PM, Reddy MM. Control of CFTR chloride conductance by ATP levels through non-hydrolytic binding. Nature 1992;360:79-81. [Crossref] [PubMed]
- Gabriel SE, Clarke LL, Boucher RC, et al. CFTR and outward rectifying chloride channels are distinct proteins with a regulatory relationship. Nature 1993;363:263-8. [Crossref] [PubMed]
- Dechecchi MC, Rolfini R, Tamanini A, et al. Effect of modulation of protein kinase C on the cAMP-dependent chloride conductance in T84 cells. FEBS Lett 1992;311:25-8. [Crossref] [PubMed]
- Berger HA, Travis SM, Welsh MJ. Regulation of the cystic fibrosis transmembrane conductance regulator Cl- channel by specific protein kinases and protein phosphatases. J Biol Chem 1993;268:2037-47. [PubMed]
- Dechecchi MC, Tamanini A, Berton G, et al. Protein kinase C activates chloride conductance in C127 cells stably expressing the cystic fibrosis gene. J Biol Chem 1993;268:11321-5. [PubMed]
- Galli F, Battistoni A, Gambari R, et al. Working Group on Inflammation in Cystic Fibrosis. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim Biophys Acta 2012;1822:690-713. [Crossref] [PubMed]
- Cabrini G, Bezzerri V, Mancini I, et al. Targeting transcription factor activity as a strategy to inhibit pro-inflammatory genes involved in cystic fibrosis: decoy oligo nucleotides and low-molecular weight compounds. Curr Med Chem 2010;17:4392-404. [Crossref] [PubMed]
- Hoenderdos K, Lodge KM, Hirst RA, et al. Hypoxia upregulates neutrophil degranulation and potential for tissue injury. Thorax 2016;71:1030-8. [Crossref] [PubMed]
- Marcos V, Zhou-Suckow Z, Önder Yildirim A, et al. Free DNA in cystic fibrosis airway fluids correlates with airflow obstruction. Mediators Inflamm 2015;2015. [Crossref] [PubMed]
- Law SM, Gray RD. Neutrophil extracellular traps and the dysfunctional innate immune response of cystic fibrosis lung disease: a review. J Inflamm (Lond) 2017;14:29. [Crossref] [PubMed]
- Gray RD, Hardisty G, Regan KH, et al. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax 2018;73:134-44. [Crossref] [PubMed]
- Tabary O, Zahm JM, Hinnrasky J, et al. Selective up-regulation of chemokine IL-8 expression in cystic fibrosis bronchial gland cells in vivo and in vitro. Am J Pathol 1998;153:921-30. [Crossref] [PubMed]
- Tirouvanziam R, de Bentzmann S, Hubeau C, et al. Inflammation and infection in naive human cystic fibrosis airway grafts. Am J Respir Cell Mol Biol 2000;23:121-7. [Crossref] [PubMed]
- Rosen BH, Evans TIA, Moll SR, et al. Infection is Not Required for Muco-inflammatory Lung Disease in CFTR-knockout Ferrets. Am J Respir Crit Care Med 2018;197:1308-18. [Crossref] [PubMed]
- Lynch JP 3rd, Sayah DM, Belperio JA, et al. Lung transplantation for cystic fibrosis: results, indications, complications, and controversies. Semin Respir Crit Care Med 2015;36:299-320. [Crossref] [PubMed]
- Chaparro C, Keshavjee S. Lung transplantation for cystic fibrosis: an update. Expert Rev Respir Med 2016;10:1269-80. [Crossref] [PubMed]
- Snell G, Reed A, Stern M, et al. The evolution of lung transplantation for cystic fibrosis: A 2017 update. J Cyst Fibros 2017;16:553-64. [Crossref] [PubMed]
- Tsui LC. The cystic fibrosis transmembrane conductance regulator gene. Am J Respir Crit Care Med 1995;151:S47-53. [Crossref] [PubMed]
- Tsui LC, Dorfman R. The cystic fibrosis gene: a molecular genetic perspective. Cold Spring Harb Perspect Med 2013;3. [Crossref] [PubMed]
- Bobadilla JL, Macek M Jr, Fine JP, et al. Cystic fibrosis: a worldwide analysis of CFTR mutations--correlation with incidence data and application to screening. Hum Mutat 2002;19:575-606. [Crossref] [PubMed]
- Cheng SH, Gregory RJ, Marshall J, et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 1990;63:827-34. [Crossref] [PubMed]
- Zielenski J, Tsui LC. Cystic fibrosis: genotypic and phenotypic variations. Annu Rev Genet 1995;29:777-807. [Crossref] [PubMed]
- Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med 2010;363:301-4. [Crossref] [PubMed]
- Illsley NP, Verkman AS. Membrane chloride transport measured using a chloride-sensitive fluorescent probe. Biochemistry 1987;26:1215-9. [Crossref] [PubMed]
- Renier M, Tamanini A, Nicolis E, et al. Use of a membrane potential-sensitive probe to assess biological expression of the cystic fibrosis transmembrane conductance regulator. Hum Gene Ther 1995;6:1275-83. [Crossref] [PubMed]
- Galietta LJ, Haggie PM, Verkman AS. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett 2001;499:220-4. [Crossref] [PubMed]
- Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov 2009;8:153-71. [Crossref] [PubMed]
- Biwersi J, Farah N, Wang YX, et al. Synthesis of cell-impermeable Cl-sensitive fluorescent indicators with improved sensitivity and optical properties. Am J Physiol 1992;262:C242-50. [PubMed]
- Biwersi J, Tulk B, Verkman AS. Long-wavelength chloride-sensitive fluorescent indicators. Anal Biochem 1994;219:139-43. [Crossref] [PubMed]
- Jayaraman S, Biwersi J, Verkman AS. Synthesis and characterization of dual-wavelength Cl-sensitive fluorescent indicators for ratio imaging. Am J Physiol 1999;276:C747-57. [Crossref] [PubMed]
- Cabrini G, Verkman AS. Potential-sensitive response mechanism of diS-C3-(5) in biological membranes. J Membr Biol 1986;92:171-82. [Crossref] [PubMed]
- Cabrini G, Verkman AS. Localization of cyanine dye binding to brush-border membranes by quenching of n-(9-anthroyloxy) fatty acid probes. Biochim Biophys Acta 1986;862:285-93. [Crossref] [PubMed]
- Van Goor F, Straley KS, Cao D, et al. Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol 2006;290:L1117-30. [Crossref] [PubMed]
- Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A 2009;106:18825-30. [Crossref] [PubMed]
- Van Goor F, Hadida S, Grootenhuis PD, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A 2011;108:18843-8. [Crossref] [PubMed]
- Jayaraman S, Haggie P, Wachter RM, et al. Mechanism and cellular applications of a green fluorescent protein-based halide sensor. J Biol Chem 2000;275:6047-50. [Crossref] [PubMed]
- Galietta LV, Jayaraman S, Verkman AS. Cell-based assay for high-throughput quantitative screening of CFTR chloride transport agonists. Am J Physiol Cell Physiol 2001;281:C1734-42. [Crossref] [PubMed]
- Galietta LJ, Springsteel MF, Eda M, et al. Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J Biol Chem 2001;276:19723-8. [Crossref] [PubMed]
- Ma T, Vetrivel L, Yang H, et al. High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J Biol Chem 2002;277:37235-41. [Crossref] [PubMed]
- Caci E, Folli C, Zegarra-Moran O, et al. CFTR activation in human bronchial epithelial cells by novel benzoflavone and benzimidazolone compounds. Am J Physiol Lung Cell Mol Physiol 2003;285:L180-8. [Crossref] [PubMed]
- Yang H, Shelat AA, Guy RK, et al. Nanomolar affinity small molecule correctors of defective Delta F508-CFTR chloride channel gating. J Biol Chem 2003;278:35079-85. [Crossref] [PubMed]
- Ma T, Thiagarajah JR, Yang H, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest 2002;110:1651-8. [Crossref] [PubMed]
- Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med 2010;363:1991-2003. [Crossref] [PubMed]
- Ramsey BW, Davies J, McElvaney NG, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663-72. [Crossref] [PubMed]
- Davies JC, Wainwright CE, Canny GJ, et al. VX08-770-103 (ENVISION) Study Group. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med 2013;187:1219-25. [Crossref] [PubMed]
- Hebestreit H, Sauer-Heilborn A, Fischer R, et al. Effects of ivacaftor on severely ill patients with cystic fibrosis carrying a G551D mutation. J Cyst Fibros 2013;12:599-603. [Crossref] [PubMed]
- Barry PJ, Plant BJ, Nair A, et al. Effects of ivacaftor in patients with cystic fibrosis who carry the G551D mutation and have severe lung disease. Chest 2014;146:152-8. [Crossref] [PubMed]
- De Boeck K, Paskavitz J, Chen X, et al. Ivacaftor, a CFTR potentiator, in cystic fibrosis patients who have a nonG551D-CFTR gating mutation: Phase 3, part 1 results. Pediatr Pulmonol 2013;48:S36.
- De Boeck K, Zolin A, Cuppens H, et al. The relative frequency of CFTR mutation classes in European patients with cystic fibrosis. J Cyst Fibros 2014;13:403-9. [Crossref] [PubMed]
- Clancy JP, Rowe SM, Accurso FJ, et al. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 2012;67:12-8. [Crossref] [PubMed]
- Boyle MP, Bell SC, Konstan MW, et al. VX09-809-102 study group. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med 2014;2:527-38. [Crossref] [PubMed]
- Wainwright CE, Elborn JS, Ramsey BW, et al. TRAFFIC Study Group. TRANSPORT Study Group. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N Engl J Med 2015;373:220-31. [Crossref] [PubMed]
- Rowe SM, McColley SA, Rietschel E, et al. VX09-809-102 Study Group. Lumacaftor/Ivacaftor Treatment of Patients with Cystic Fibrosis Heterozygous for F508del-CFTR. Ann Am Thorac Soc 2017;14:213-9. [PubMed]
- Cholon DM, Quinney NL, Fulcher ML, et al. Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis. Sci Transl Med 2014;6. [Crossref] [PubMed]
- Veit G, Avramescu RG, Perdomo D, et al. Some gating potentiators, including VX-770, diminish ΔF508-CFTR functional expression. Sci Transl Med 2014;6. [Crossref] [PubMed]
- Matthes E, Goepp J, Carlile GW, et al. Low free drug concentration prevents inhibition of F508del CFTR functional expression by the potentiator VX – 770 (ivacaftor). Br J Pharmacol 2016;173:459-70. [Crossref] [PubMed]
- Hanrahan JW, Matthes E, Carlile G, et al. Corrector combination therapies for F508del-CFTR. Curr Opin Pharmacol 2017;34:105-11. [Crossref] [PubMed]
- Howard M, Frizzell RA, Bedwell DM. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat Med 1996;2:467-9. [Crossref] [PubMed]
- Barton-Davis ER, Cordier L, Shoturma DI, et al. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J Clin Invest 1999;104:375-81. [Crossref] [PubMed]
- Wilschanski M, Famini C, Blau H, et al. A pilot study of the effect of gentamicin on nasal potential difference measurements in cystic fibrosis patients carrying stop mutations. Am J Respir Crit Care Med 2000;161:860-5. [Crossref] [PubMed]
- Clancy JP, Bebök Z, Ruiz F, et al. Evidence that systemic gentamicin suppresses premature stop mutations in patients with cystic fibrosis. Am J Respir Crit Care Med 2001;163:1683-92. [Crossref] [PubMed]
- Wilschanski M, Yahav Y, Yaacov Y, et al. Gentamicin-induced correction of CFTR function in patients with cystic fibrosis and CFTR stop mutations. N Engl J Med 2003;349:1433-41. [Crossref] [PubMed]
- Linde L, Boelz S, Nissim-Rafinia M, et al. Nonsense-mediated mRNA decay affects nonsense transcript levels and governs response of cystic fibrosis patients to gentamicin. J Clin Invest 2007;117:683-92. [Crossref] [PubMed]
- Kerem E, Hirawat S, Armoni S, et al. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: a prospective phase II trial. Lancet 2008;372:719-27. [Crossref] [PubMed]
- Sermet-Gaudelus I, Boeck KD, Casimir GJ, et al. Ataluren (PTC124) induces cystic fibrosis transmembrane conductance regulator protein expression and activity in children with nonsense mutation cystic fibrosis. Am J Respir Crit Care Med 2010;182:1262-72. [Crossref] [PubMed]
- Wilschanski M, Miller LL, Shoseyov D, et al. Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur Respir J 2011;38:59-69. [Crossref] [PubMed]
- Kerem E, Konstan MW, De Boeck K, et al. Cystic Fibrosis Ataluren Study Group. Ataluren for the treatment of nonsense-mutation cystic fibrosis: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med 2014;2:539-47. [Crossref] [PubMed]
- Zainal Abidin N, Haq IJ, Gardner AI, et al. Ataluren in cystic fibrosis: development, clinical studies and where are we now? Expert Opin Pharmacother 2017;18:1363-71. [Crossref] [PubMed]
- Liessi N, Cichero E, Pesce E, et al. Synthesis and biological evaluation of novel thiazole- VX-809 hybrid derivatives as F508del correctors by QSAR-based filtering tools. Eur J Med Chem 2018;144:179-200. [Crossref] [PubMed]
- Wang X, Liu B, Searle X, et al. Discovery of 4-[(2R,4R)-4-({[1-(2,2-Difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl}amino)-7-(difluoromethoxy)-3,4-dihydro-2H-chromen-2-yl]benzoic Acid (ABBV/GLPG-2222), a Potent Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Corrector for the Treatment of Cystic Fibrosis. J Med Chem 2018;61:1436-49. [Crossref] [PubMed]
- Pesci E, Bettinetti L, Fanti P, et al. Novel Hits in the Correction of ΔF508-Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Protein: Synthesis, Pharmacological, and ADME Evaluation of Tetrahydropyrido[4,3-d]pyrimidines for the Potential Treatment of Cystic Fibrosis. J Med Chem 2015;58:9697-711. [Crossref] [PubMed]
- Cendret V, Legigan T, Mingot A, et al. Synthetic deoxynojirimycin derivatives bearing a thiolated, fluorinated or unsaturated N-alkyl chain: identification of potent α-glucosidase and trehalase inhibitors as well as F508del-CFTR correctors. Org Biomol Chem 2015;13:10734-44. [Crossref] [PubMed]
- Pesce E, Bellotti M, Liessi N, et al. Synthesis and structure-activity relationship of aminoarylthiazole derivatives as correctors of the chloride transport defect in cystic fibrosis. Eur J Med Chem 2015;99:14-35. [Crossref] [PubMed]
- Ye L, Hu B, El-Badri F, et al. ΔF508-CFTR correctors: synthesis and evaluation of thiazole-tethered imidazolones, oxazoles, oxadiazoles, and thiadiazoles. Bioorg Med Chem Lett 2014;24:5840-4. [Crossref] [PubMed]
- Coffman KC, Nguyen HH, Phuan PW, et al. Constrained bithiazoles: small molecule correctors of defective ΔF508-CFTR protein trafficking. J Med Chem 2014;57:6729-38. [Crossref] [PubMed]
- Favia M, Mancini MT, Bezzerri V, et al. Trimethylangelicin promotes the functional rescue of mutant F508del CFTR protein in cystic fibrosis airway cells. Am J Physiol Lung Cell Mol Physiol 2014;307:L48-61. [Crossref] [PubMed]
- Phuan PW, Veit G, Tan J, et al. Synergy-based small-molecule screen using a human lung epithelial cell line yields ΔF508-CFTR correctors that augment VX-809 maximal efficacy. Mol Pharmacol 2014;86:42-51. [Crossref] [PubMed]
- Compain P, Decroocq C, Joosten A, et al. Rescue of functional CFTR channels in cystic fibrosis: a dramatic multivalent effect using iminosugar cluster-based correctors. Chembiochem 2013;14:2050-8. [Crossref] [PubMed]
- Odolczyk N, Fritsch J, Norez C, et al. Discovery of novel potent ΔF508-CFTR correctors that target the nucleotide binding domain. EMBO Mol Med 2013;5:1484-501. [Crossref] [PubMed]
- Phuan PW, Yang B, Knapp JM, et al. Cyanoquinolines with independent corrector and potentiator activities restore ΔPhe508-cystic fibrosis transmembrane conductance regulator chloride channel function in cystic fibrosis. Mol Pharmacol 2011;80:683-93. [Crossref] [PubMed]
- Pedemonte N, Tomati V, Sondo E, et al. Dual activity of aminoarylthiazoles on the trafficking and gating defects of the cystic fibrosis transmembrane conductance regulator chloride channel caused by cystic fibrosis mutations. J Biol Chem 2011;286:15215-26. [Crossref] [PubMed]
- Kalid O, Mense M, Fischman S, et al. Small molecule correctors of F508del-CFTR discovered by structure-based virtual screening. J Comput Aided Mol Des 2010;24:971-91. [Crossref] [PubMed]
- Kim Chiaw P, Wellhauser L, Huan LJ, et al. A chemical corrector modifies the channel function of F508del-CFTR. Mol Pharmacol 2010;78:411-8. [Crossref] [PubMed]
- Ye L, Knapp JM, Sangwung P, et al. Pyrazolylthiazole as DeltaF508-cystic fibrosis transmembrane conductance regulator correctors with improved hydrophilicity compared to bithiazoles. J Med Chem 2010;53:3772-81. [Crossref] [PubMed]
- Yu GJ, Yang B, Verkman AS, et al. Isoxazolopyrimidines as Novel ΔF508-CFTR Correctors. Synlett 2010;2010:1063-6. [Crossref] [PubMed]
- Wellhauser L, Kim Chiaw P, Pasyk S, et al. A small-molecule modulator interacts directly with deltaPhe508-CFTR to modify its ATPase activity and conformational stability. Mol Pharmacol 2009;75:1430-8. [Crossref] [PubMed]
- Yu GJ, Yoo CL, Yang B, et al. Potent s-cis-locked bithiazole correctors of DeltaF508 cystic fibrosis transmembrane conductance regulator cellular processing for cystic fibrosis therapy. J Med Chem 2008;51:6044-54. [Crossref] [PubMed]
- Yoo CL, Yu GJ, Yang B, et al. 4'-Methyl-4,5'-bithiazole-based correctors of defective delta F508-CFTR cellular processing. Bioorg Med Chem Lett 2008;18:2610-4. [Crossref] [PubMed]
- Suen YF, Robins L, Yang B, et al. Sulfamoyl-4-oxoquinoline-3-carboxamides: novel potentiators of defective DeltaF508-cystic fibrosis transmembrane conductance regulator chloride channel gating. Bioorg Med Chem Lett 2006;16:537-40. [Crossref] [PubMed]
- Pedemonte N, Diena T, Caci E, et al. Antihypertensive 1,4-dihydropyridines as correctors of the cystic fibrosis transmembrane conductance regulator channel gating defect caused by cystic fibrosis mutations. Mol Pharmacol 2005;68:1736-46. [PubMed]
- Pedemonte N, Lukacs GL, Du K, et al. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest 2005;115:2564-71. [Crossref] [PubMed]
- Pedemonte N, Sonawane ND, Taddei A, et al. Phenylglycine and sulfonamide correctors of defective delta F508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol Pharmacol 2005;67:1797-807. [Crossref] [PubMed]
- Taylor-Cousar JL, Munck A, McKone EF, et al. Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N Engl J Med 2017;377:2013-23. [Crossref] [PubMed]
- Rowe SM, Daines C, Ringshausen FC, et al. Tezacaftor-Ivacaftor in Residual-Function Heterozygotes with Cystic Fibrosis. N Engl J Med 2017;377:2024-35. [Crossref] [PubMed]
- Donaldson SH, Pilewski JM, Griese M, et al. VX11-661-101 Study Group. Tezacaftor/Ivacaftor in Subjects with Cystic Fibrosis and F508del/F508del-CFTR or F508del/G551D-CFTR. Am J Respir Crit Care Med 2018;197:214-24. [Crossref] [PubMed]
- Dalemans W, Barbry P, Champigny G, et al. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature 1991;354:526-8. [Crossref] [PubMed]
- Phuan PW, Veit G, Tan JA, et al. Potentiators of Defective ΔF508-CFTR Gating that Do Not Interfere with Corrector Action. Mol Pharmacol 2015;88:791-9. [Crossref] [PubMed]
- Moss RB, Flume PA, Elborn JS, et al. VX11-770-110 (KONDUCT) Study Group. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117His-CFTR mutation: a double-blind, randomised controlled trial. Lancet Respir Med 2015;3:524-33. [Crossref] [PubMed]
- Fernandez Alanis E, Pinotti M, Dal Mas A, et al. An exon-specific U1 small nuclear RNA (snRNA) strategy to correct splicing defects. Hum Mol Genet 2012;21:2389-98. [Crossref] [PubMed]
- Sharma M, Benharouga M, Hu W, et al. Conformational and temperature-sensitive stability defects of the delta F508 cystic fibrosis transmembrane conductance regulator in post-endoplasmic reticulum compartments. J Biol Chem 2001;276:8942-50. [Crossref] [PubMed]
- Okiyoneda T, Barrière H, Bagdány M, et al. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 2010;329:805-10. [Crossref] [PubMed]
- Fu L, Rab A, Tang LP, et al. ΔF508 CFTR surface stability is regulated by DAB2 and CHIP-mediated ubiquitination in post-endocytic compartments. PLoS One 2015;10. [Crossref] [PubMed]
- Meng X, Clews J, Kargas V, et al. The cystic fibrosis transmembrane conductance regulator (CFTR) and its stability. Cell Mol Life Sci 2017;74:23-38. [Crossref] [PubMed]
- Guerra L, Fanelli T, Favia M, et al. Na+/H+ exchanger regulatory factor isoform 1 overexpression modulates cystic fibrosis transmembrane conductance regulator (CFTR) expression and activity in human airway 16HBE14o- cells and rescues DeltaF508 CFTR functional expression in cystic fibrosis cells. J Biol Chem 2005;280:40925-33. [Crossref] [PubMed]
- Favia M, Guerra L, Fanelli T, et al. Na+/H+ exchanger regulatory factor 1 overexpression-dependent increase of cytoskeleton organization is fundamental in the rescue of F508del cystic fibrosis transmembrane conductance regulator in human airway CFBE41o- cells. Mol Biol Cell 2010;21:73-86. [Crossref] [PubMed]
- Rubino R, Bezzerri V, Favia M, et al. Pseudomonas aeruginosa reduces the expression of CFTR via post-translational modification of NHERF1. Pflugers Arch 2014;466:2269-78. [Crossref] [PubMed]
- Rowe SM, Verkman AS. Cystic fibrosis transmembrane regulator correctors and potentiators. Cold Spring Harb Perspect Med 2013;3. [Crossref] [PubMed]
- Mills AD, Yoo C, Butler JD, et al. Design and synthesis of a hybrid potentiator-corrector agonist of the cystic fibrosis mutant protein DeltaF508-CFTR. Bioorg Med Chem Lett 2010;20:87-91. [Crossref] [PubMed]
- Collawn JF, Fu L, Bartoszewski R, et al. Rescuing ΔF508 CFTR with trimethylangelicin, a dual-acting corrector and potentiator. Am J Physiol Lung Cell Mol Physiol 2014;307:L431-4. [Crossref] [PubMed]
- Knapp JM, Wood AB, Phuan PW, et al. Structure-activity relationships of cyanoquinolines with corrector-potentiator activity in ΔF508 cystic fibrosis transmembrane conductance regulator protein. J Med Chem 2012;55:1242-51. [Crossref] [PubMed]
- Tamanini A, Borgatti M, Finotti A, et al. Trimethylangelicin reduces IL-8 transcription and potentiates CFTR function. Am J Physiol Lung Cell Mol Physiol 2011;300:L380-90. [Crossref] [PubMed]
- Liu J, Bihler H, Farinha CM, et al. Partial rescue of F508del-CFTR channel gating with modest improvement of protein processing, but not stability by a dual-acting small molecule. Br J Pharmacol 2018;175:1017-38. [Crossref] [PubMed]
- Laselva O, Molinski S, Casavola V, et al. The investigational Cystic Fibrosis drug Trimethylangelicin directly modulates CFTR by stabilizing the first membrane-spanning domain. Biochem Pharmacol 2016;119:85-92. [Crossref] [PubMed]
- Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J Clin Invest 1997;100:2457-65. [Crossref] [PubMed]
- Rubenstein RC, Zeitlin PL. A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in deltaF508-homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. Am J Respir Crit Care Med 1998;157:484-90. [Crossref] [PubMed]
- Chung WJ, Goeckeler-Fried JL, Havasi V, et al. Increasing the Endoplasmic Reticulum Pool of the F508del Allele of the Cystic Fibrosis Transmembrane Conductance Regulator Leads to Greater Folding Correction by Small Molecule Therapeutics. PLoS One 2016;11. [Crossref] [PubMed]
- Molinski SV, Ahmadi S, Ip W, et al. Orkambi® and amplifier co-therapy improves function from a rare CFTR mutation in gene-edited cells and patient tissue. EMBO Mol Med 2017;9:1224-43. [Crossref] [PubMed]
- Fabbri E, Tamanini A, Jakova T, et al. A Peptide Nucleic Acid against MicroRNA miR-145-5p Enhances the Expression of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in Calu-3 Cells. Molecules 2017;23. [Crossref] [PubMed]
- Veit G, Bossard F, Goepp J, et al. Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol Biol Cell 2012;23:4188-202. [Crossref] [PubMed]
- Ruffin M, Roussel L, Maillé É, et al. Vx-809/Vx-770 treatment reduces inflammatory response to Pseudomonas aeruginosa in primary differentiated cystic fibrosis bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2018;314:L635-41. [Crossref] [PubMed]
- Dechecchi MC, Nicolis E, Norez C, et al. Anti-inflammatory effect of miglustat in bronchial epithelial cells. J Cyst Fibros 2008;7:555-65. [Crossref] [PubMed]
- Lampronti I, Manzione MG, Sacchetti G, et al. Differential Effects of Angelicin Analogues on NF-κB Activity and IL-8 Gene Expression in Cystic Fibrosis IB3-1 Cells. Mediators Inflamm 2017;2017. [Crossref] [PubMed]
- Döring G, Flume P, Heijerman H, et al. Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros 2012;11:461-79. [Crossref] [PubMed]
- Nichols DP, Chmiel JF. Inflammation and its genesis in cystic fibrosis. Pediatr Pulmonol 2015;50:S39-56. [Crossref] [PubMed]
- Torphy TJ, Allen J, Cantin AM, et al. Anti-inflammatory Therapy Working Group. Considerations for the Conduct of Clinical Trials with Anti-inflammatory Agents in Cystic Fibrosis. A Cystic Fibrosis Foundation Workshop Report. Ann Am Thorac Soc 2015;12:1398-406. [Crossref] [PubMed]
- Cantin AM, Hartl D, Konstan MW, et al. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J Cyst Fibros 2015;14:419-30. [Crossref] [PubMed]


