Should we titrate peep based on end-expiratory transpulmonary pressure?—yes
Introduction
Application of basic physiologic principles has revolutionized the treatment of patients with acute respiratory distress syndrome (ARDS). Current standard of care limits tidal volumes (VT) to 6 cc/kg, keeps end-inspiratory plateau pressures below 30 cmH2O and provides adequate positive end expiratory pressures (PEEP) to keep the lung open (1-6). At this time there remains no consensus as to the best method to determine optimal PEEP for a patient despite the importance of this intervention (7,8). The use of esophageal manometry has helped form the foundation of our current understanding of pulmonary pathophysiology (9-12), and over the last two decades has moved from basic physiology research to clinical care in the intensive care unit, allowing optimization of PEEP levels (13,14). Esophageal pressures (PES) serve as an estimate of pleural pressures (PPL), thus differentiating the mechanics of the lung itself (transpulmonary pressure—PL) from the chest wall (including the rib cage, diaphragm and abdomen—PCW) (10-12). As ventilator induced lung injury is caused by the distending pressures applied to the lung itself, with highly variable chest wall characteristics among patients, controlling airway pressure alone is inadequate and potentially harmful. Esophageal manometry allows for a more personalized approach to ventilator management and has become routinely used in patients with ARDS where precise and careful differentiation of chest wall and lung pressures and optimization of the ventilator may lead to improved mechanics, oxygenation and outcomes (13,14). In particular esophageal manometry used to estimate transpulmonary pressures has become used to set the positive end-expiratory pressures (PEEP).
Optimal PEEP is thought to balance several important principles; avoiding overly high pressures which could overdistend the lung causing barotrauma, inflammation and hemodynamic compromise (15-17), while improving oxygenation and mechanics by preventing lung derecruitment (collapse), increasing functional lung volumes, decreasing cyclic opening and closing and the airways (shear stresses from atelectrauma) (15-17). Although the “open lung” strategy (characterized by a recruitment maneuver and decremental PEEP titration strategy) has recently been challenged after a study revealed an increase in mortality, this study did not use PEEP titration to esophageal pressures, and used a non-standard recruitment maneuver strategy (18). As such, this study likely has minimal implications on the real world application of “open lung” strategies aiming to prevent derecruitment, especially in regards to the use of esophageal manometry to set PEEP.
As negative transpulmonary pressure may lead to lung or airway collapse, adjusting PEEP to achieve positive end-expiratory transpulmonary pressures prevents collapse and optimizes lung mechanics (13,14,19-21). Esophageal pressure measurements thereby serve several purposes in ARDS. First, these measurements can be used to adjust PEEP to counter the baseline elevated pleural pressures found in patients with obesity, elevated abdominal pressures and critical illness that would otherwise result in negative transpulmonary pressures and lung collapse. Second, transpulmonary measurements may be used monitor for lung overdistension and to account for the stiffened chest wall during tidal breathing which may impact forces across the lungs. Additionally, other uses of PES in the ICU include monitoring of dyssynchrony, estimation and adjustment for auto-PEEP and assisting with weaning, but have been covered in depth in other reviews (22-25).
Although the tidal fluctuations in PES (∆PES) are widely agreed to represent the changes in pleural pressure for estimation of the lung and chest wall compliance, the interpretation of the actual pressure values (also known as the “absolute” pressures) measured by esophageal manometry have caused some disagreement and controversy (26-34). This debate has led further to differences in clinical application as both new definitions and incorrect assumptions have clouded the discussion (32). As such, this article will review the evidence for using the actual value of esophageal pressure to estimate pleural pressures as well as the background, rationale and evidence for use of esophageal manometry to set PEEP for clinical care in patients with ARDS, while addressing some of the controversies associated with this subject.
Pressure definitions and rationale for use
The definitions of the relevant pressures were first described by classic respiratory physiologists in the 1950’s (9,10,35), and it is important to define and understand these pressures when reviewing the literature as there have been several misconceptions introduced which obfuscate the correct application and interpretation. As we have previously reviewed (22), the pressure across the respiratory system (PRS) is defined as the difference between the pressure at the airway (PAO) and body surface (PBS) (PRS = PAO − PBS). The pressure difference across the lung is called the transpulmonary pressure (PL) and is the difference between PAO and pleural pressure (PPL) (PL = PAO − PPL). Finally the pressure across the chest wall (PCW) is defined as the PPL minus PBS (PCW = PPL − PBS) (11). Esophageal balloon pressures (PES) represent central thorax pressures but despite some regional and positional variability have been determined to be a good surrogate for average “effective” pleural pressures as we will review (36-40).
Critically ill patients frequently have elevated pleural pressures due to abdominal distension, abdominal hypertension, pulmonary and chest wall edema and pleural effusion (Figure 1) (13,40,41). Particularly in the heterogeneously injured lung, these elevated pleural pressures may be higher than the local alveolar and airway pressures causing regions of lung collapse. Collapse, caused from negative transpulmonary pressures, can lead to decreased aeration, worsened oxygenation and worsened pulmonary mechanics with decreased functional lung size secondary to closed airways and flooded lung units. The elevated pleural pressures causing this collapse can be detected and measured as an elevated PES and negative PL (Figure 1). Our group and others have noted that transpulmonary pressures were frequently negative at end-expiration in patients with ARDS (13,40). As this suggests that airway collapse or flooding has prevented alveolar pressures from equilibrating with airway pressures, it was inferred that PEEP could be adjusted above these closing pressures (measured by PES) to keep airways open at end-expiration.
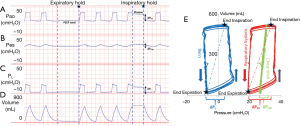
Evidence supporting use of the actual value of PES
The use of PES to estimate PPL has been well established in human and animal studies over the past seventy years (9,10,35). Animal studies in the 1970s using direct pleural pressure assessment suggested that PES closely mirrored the lateral mid-thoracic pressures during direct measurement (42). More recent animal studies have confirmed these findings in a canine ARDS model to examine the relationship between measured esophageal pressures and directly measured pleural pressures (38). Using wafer pressure sensors in non-dependent, mid-thoracic and dependent lung regions, and testing at multiple levels of PEEP and tidal volumes Pelosi et al. found that mid thoracic PPL closely matched measured PES at low lung volumes (38). Additionally as expected they confirmed overestimation of pleural pressures in the non-dependent regions (by roughly 7 cmH2O) and underestimation of pleural pressures I the non-dependent regions (by roughly 4 cmH2O) (38).
The dependent and non-dependent variation that Pelosi and others have confirmed has led to some confusion in application as there is no single value of pleural pressure. Furthermore, as lung collapse or spatial distortion of the chest wall may cause local changes in PPL, there was some concern about using PES as a global average for PPL. These concerns were investigated in a model of chest wall and lung distortion in rats and confirmed that PES could be used to estimate average PPL in both normal and deformed lungs (39). Similarly in patients with acute lung injury, PES was used to infer an average (or effective) transpulmonary pressure in a physiology experiment measuring gastric, bladder, esophageal pressures and transpulmonary pressures (20,40). More recently, Yoshida et al. showed in both pigs and human cadavers, that expiratory and inspiratory transpulmonary pressures using PES closely reflected the values obtained via direct measurement of the pleural pressures in dependent and mid lung zones (36). PES was measured over a wide range of PEEP levels with PPL simultaneously measured in non-dependent and dependent regions of the pleura. These data lend further support to using the actual value of PES as a surrogate for PPL as the best correlation seems to be in regions of lung most sensitive to collapse at end expiration (36).
Although the actual value of PES does seem to reflect measured PPL, the relationship between PES and PPL was initially described in upright, spontaneously breathing patients in the study of classic respiratory physiology (11). When patients are moved into the supine position (as are our critically ill ARDS patients), the balloon sits directly under the weight of the mediastinum, and abdominal contents push upwards against the diaphragm raising the measured value of PES (37). Roughly 3–7 cmH2O of additional pressure is thought to be due to positioning when correcting the PES to estimate the effective PPL with the supine position causing increased pressure from a decrease in lung volume and the shift in mediastinal weight (37). As such we usually consider this to cause on average a 5 cmH2O artifact in our measurements. This estimated artifact is in agreement with a recent study comparing ex vivo measurements pre-lung transplant with in vivo measurements of the same lungs post-transplant (43). This elegant study found PPL was roughly 5 cmH2O less than the measured PES which if used clinically would provide roughly 5 cmH2O additional transpulmonary pressure if PEEP was titrated to equal the measured PES (44).
Lastly there has been some concern about the validity of PES, due to the frequently positive values measured (7,28-30,33). Some have worried that this would not be compatible with an open lung, and their concern led to an alternative calculation of PL. This alternative is the “elastance based” method of calculating PL [PL = PAO × lung elastance (EL)/respiratory system elastance (ERS)] (26). ∆PES during tidal breathing is used to estimate ∆PL and then calculate EL as ∆PL/VT. The “elastance based” technique assumes pleural pressure at end expiration to be zero (atmospheric) when airway pressure is zero (26). This alternative definition measures the cyclical stress (∆PL) during tidal breathing, but does not account for the baseline PL, which may be widely variable and is usually not equal to atmospheric pressures. As pleural pressures are often very elevated (due to obesity, abdominal pathologies causing elevated pressures or edema/effusions), assuming a pleural pressure of zero in this alternative approach is clearly incorrect (32) and will result in significantly different estimations of transpulmonary pressures (31).
In patients with obesity or elevated abdominal pressure, a larger pressure is needed to displace the diaphragm and abdomen during inflation. This is because the pressure volume curve of the chest wall is shifted to higher pressures, but not necessarily because the chest wall has become less compliant (32). Therefore in the supine position the absolute lung volumes and PL can be significantly lower than in normal patients. Importantly, positive pleural pressures (and hence negative PL) do not need to be compatible with an open lung as elevated PPL may cause airway collapse preventing communication of alveoli with the upper airway (gas trapping) at end expiration (32). PPL and alveolar pressure (PALV) may be substantially increased above atmospheric pressure at end expiration secondary to small airway collapse in obese patients or alveolar flooding in acute respiratory distress syndrome (ARDS). Indeed Behazin et al. showed that airway pressures must often be raised until greater than the measured PES in order for inspiratory flow and volume gain to begin in obese patients (40). They also showed a positive correlation between the PES at relaxed volume and the airway pressure needed to initiate flow further illustrating this relationship (40). Additionally our own unpublished data using slow-flow pressure-volume (PV) loops and trials of zero PEEP in patients with obesity and abdominal pathologies emphasizes this point (Figure 2). The figure illustrates that there is zero flow or volume gain from the initiation of the PV loop when airway pressure is zero until the pressures overcome the elevated PPL (as measured by PES). In these same patients, while on zero PEEP, we often measure substantial intrinsic positive end-expiratory pressure (PEEPi) during expiratory breath holds. This PEEPi is close in value to both the measured PES during holds and the airway pressure required to overcome pleural pressure on the PV loop and appear to be caused by elevated PPL causing airway collapse (Figure 2). In further agreement with these findings, Fumagalli et al. used electric impedance tomography to show that lung collapse begins when PES approaches and then overcomes the airway pressures leading to low-negative PL (41).
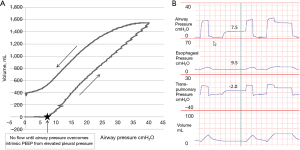
In conclusion, despite the minor limitations illustrated with positional gravitational artifact, PES seems to be a good estimate for the “effective” PPL in multiple human and animal studies in normal patients, the obese, and patients with ARDS. The inaccuracies in estimating PPL from PES in an individual patient appear minor compared with the differences in PES between different patients. Additionally positive PES values in some patients appear to not be “artifact” as some have claimed, but to actually be another valuable piece of clinical information that should not be ignored or discounted (20). Proper inflation (45), and balloon placement (46) are needed for correct interpretation, but these can be easily learned for proper implementation (47).
Evidence supporting the use of PES to set PEEP
If we can accept that the actual value of the PES reflects an “effective” PPL, than the benefit for using these values clinically becomes obvious. As we have previously illustrated the goal of PEEP is to prevent derecruitment, maintain alveolar aeration and improve the functional size of the “baby lung”. If pleural pressures are higher than the applied PEEP producing a negative end-expiratory transpulmonary pressure (Figure 3), this will encourage collapse in midzone and dependent lung regions. Elevated pleural pressures are found for numerous reasons including edema, pleural effusions, elevated abdominal pressures from obesity and abdominal pathologies with pressures transmitted across the diaphragm, and increasing PEEP to match these pressures (Figure 3) will improve this lung collapse. In fact, Malbrain et al. found that the majority of patients in the ICU had elevated abdominal pressures (57% had pressures greater than 16 cmH2O, and 21% were over 21 cmH2O) (48). Unfortunately there does not appear to be a consistent way to estimate which patients have elevated PPL to determine who would empirically benefit from higher levels of PEEP. Indeed our group found a significant number of patients with elevated end-expiratory pleural pressures (17.5 cmH2O on average) without significant correlation to either body mass index or chest wall elastance (13). While there was correlation between PEEP and end-expiratory PL, only 24% of the variance was explained by airway pressures while 52% of the PL variance was due to PES (13).
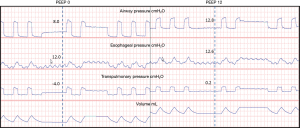
After discovering the frequency of elevated pleural pressures in the critical ill and without a clear method to differentiate empirically, a randomized controlled trial to further investigate the routine use of esophageal manometry in patients with ARDS was initiated. Talmor and colleagues in 2008 published a single center randomized controlled trial comparing standard of care lung protective ventilation with a strategy of titrating PEEP to achieve a positive PL (14). This strategy resulted in significantly higher levels of PEEP, improved oxygenation and compliance, and a strong trend towards improved survival and shorter duration of mechanical ventilation when compared with standard ARDSnet ventilation strategies (Figure 4) (14). Titrating PEEP to a positive PL may have prevented significant end-expiratory collapsing pressures, increased the size of functional lung, reduced cyclical opening and closing of the lung (atelectrauma), improved oxygenation, and prevented ventilator induced lung injury. This study remains the largest and best quality study to date, but is currently being repeated as a large multicenter study (19). The rationale for following the actual PES values to set PEEP was tested again in an animal study using surfactant depleted rats (21). The investigators found that a strategy targeting positive end-expiratory PL maintained lung volumes, improved compliance, reduced hypoxemia and pulmonary edema and decreased pro-inflammatory mediator release as well as histological evidence of ventilation induced lung injury (VILI) (21). Interestingly another study found that optimal PEEP was the same when titrated to either zero-transpulmonary pressure or to optimal intratidal gas distribution on electric impedance tomography (EIT) which might suggest that maintaining a positive end-expiratory PL prevents lung collapse and improves ventilation (49).
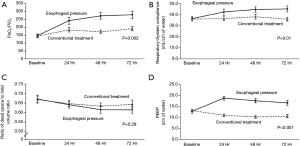
Several other recent studies further support these concepts focusing on the effects of transpulmonary pressure monitoring in obesity (41,50). Using both obese human patients as well as a swine model for obesity, it was discovered that low to negative transpulmonary pressures measured using the actual values of PES predicted lung collapse and intratidal opening and closing (41). Additionally this same group showed that PEEP titrated to transpulmonary pressures resulted in similar PEEP that was titrated to best PEEP determined by decremental PEEP trial (50). Additionally titrated PEEP (preceded by a recruitment maneuver) resulted in improved lung volumes, oxygenation and respiratory system elastance (50). To lend further support to application in the obese, this group presented an interesting case report using these strategies to help extubate a difficult to wean morbidly obese patient, further illustrating the clinical application (51). Applying higher levels of PEEP to match and counterbalance high levels of intrinsic PEEP often seen associated with obesity may result in reducing work of breathing and preventing both atelectasis and tidal recruitment-derecruitment. As we know that it is the pleural pressure that increases in these patients and not the actual stiffness of the chest wall, when individualizing PEEP to a patient it is clearly inappropriate to rely only on measurements of elastance as is done with the “elastance-based” method (32).
Of note, the alternative “elastance-based” method for PL estimation has also been used to set PEEP and guide treatment, but there is only very limited clinical data supporting its use. In a report by Grasso and colleagues in patients with severe ARDS due to influenza during the 2009 H1N1 epidemic, clinicians used the elastance-based method to avoid ECMO in seven patients with severe hypoxemia (52). In this report, they raised PEEP beyond levels that had previously been considered safe using elastance-based PL (52). Although there have been several physiology based review articles endorsing the “elastance-base” technique there is in fact very little clinical evidence to support this alternative definition. Directly comparing the traditional approach to this alternative found vastly different estimations of transpulmonary pressures (31) which would lead to substantial differences in clinical care, and the issues with this alternative approach have been addressed at length in other articles (32). As such we cannot at this time support clinical application of the “elastance-based” technique.
Using PES to limit overdistension
An additional benefit to titration of PEEP based on PES, is the improved ability to monitor and limit both cyclic and total overdistension of the lung. It has been suggested that respiratory system driving pressures may the best predictor for mortality in patients with ARDS (53), and our group proposed that the most component of these findings may be in specifically limiting the distending pressures or cyclical stress across the lungs (the transpulmonary driving pressure (∆PL) (54). We tested this hypothesis retrospectively and found that survivors had decreased transpulmonary driving pressure (55). A ∆PL of 20 cmH2O has been shown to raise healthy lungs to its total lung capacity (TLC) and continuous ventilation at TLC in animal models can lead to lethal ventilator induced lung injury (56). It has been suggested ∆PL be kept less than 10–12 cmH2O to prevent lung injury (23), as inhomogeneous lung can significantly increase local stress raiser (more than doubling or tripling local pressure) (12,57). Additionally, total lung stress (the ∆PL, additive to the static stress at end-expiratory PL) should likely be limited to less than 20–25 cmH2O. Limiting this static stress may decrease overdistension and prevent ventilator induced lung injury by keeping the total strain <2 (58). Using PES to set PEEP allows for the additional benefit of monitoring both the cyclical stress and total stress and assuring that the PEEP is not resulting in overly high pressures. With widely variable chest wall pressures and elastance, we cannot predict if we are reaching these thresholds without the use of an esophageal balloon.
Conclusions
As illustrated, PES clearly can be used to estimate “effective” pleural pressures, and there appears to be good clinical evidence for using this to set PEEP in patients with ARDS. Despite this clear clinical utility, there have been several barriers to widespread clinical adoption in setting PEEP levels. The introduction of the alternative “elastance-based” method to define PL and PES in the clinical and research literature has led to confusion and disagreement in application. The incorrect application of these principles may lead to variable or even harmful outcomes for patients. The application of PES to set PEEP levels relies upon complex physiologic principles which may discourage routine use without proper education. Although in our experience, esophageal balloon use is easily learned, proper technique in placement and interpretation is required for successful use. Of note however Norisue et al. showed in a recent paper that clinician education easily improves the ability to utilize PES measurements and suggests that more widespread application would be easy to implement.
Despite these hurdles, esophageal manometry following PES as a surrogate for PPL is easy to use and has extensive application in patient with ARDS to help improve PEEP titration. Monitoring transpulmonary and chest wall pressures uncovers the unique physiology of a given patient, personalizing care and potentially improving the patients’ outcomes. Using transpulmonary pressures to set PEEP levels may prevent derecruitment, prevent lung collapse, decrease atelectrauma caused by tidal recruitment and derecruitment, improve oxygenation, and improve lung mechanics and ventilator induced lung injury. Use of PES to target PEEP may allow for clinicians to deliver higher pressures than would not routinely be given while assuring that the lungs are not being overdistended with resulting barotrauma. Only a few interventions in ARDS have demonstrated benefit for patients, and these benefits have primarily been due to improved understanding of basic respiratory physiology. Use of the esophageal balloon for clinical care furthers our understanding of a patient’s unique pathophysiology providing a more personalized approach to our critically ill patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347-54. [Crossref] [PubMed]
- Gattinoni L, Quintel M. Is mechanical ventilation a cure for ARDS? Intensive Care Med 2016;42:916-7. [Crossref] [PubMed]
- Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:637-45. [Crossref] [PubMed]
- Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:646-55. [Crossref] [PubMed]
- Villar J, Kacmarek RM, Perez-Mendez L, et al. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 2006;34:1311-8. [Crossref] [PubMed]
- Chiumello D, Cressoni M, Carlesso E, et al. Bedside selection of positive end-expiratory pressure in mild, moderate, and severe acute respiratory distress syndrome. Crit Care Med 2014;42:252-64. [Crossref] [PubMed]
- Hess DR. Recruitment Maneuvers and PEEP Titration. Respir Care 2015;60:1688-704. [Crossref] [PubMed]
- Dornhorst AC, Leathart GL. A method of assessing the mechanical properties of lungs and air-passages. Lancet 1952;2:109-11. [Crossref] [PubMed]
- Mead J, Gaensler EA. Esophageal and pleural pressures in man, upright and supine. J Appl Physiol 1959;14:81-3. [Crossref] [PubMed]
- Mead J. Mechanical properties of lungs. Physiol Rev 1961;41:281-330. [Crossref] [PubMed]
- Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970;28:596-608. [Crossref] [PubMed]
- Talmor D, Sarge T, O'Donnell CR, et al. Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med 2006;34:1389-94. [Crossref] [PubMed]
- Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008;359:2095-104. [Crossref] [PubMed]
- Luecke T, Pelosi P. Clinical review: Positive end-expiratory pressure and cardiac output. Crit Care 2005;9:607-21. [Crossref] [PubMed]
- Suter PM, Fairley B, Isenberg MD. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med 1975;292:284-9. [Crossref] [PubMed]
- Eisner MD, Thompson BT, Schoenfeld D, et al. Airway pressures and early barotrauma in patients with acute lung injury and acute respiratory distress syndrome. Am J Respir Crit Care Med 2002;165:978-82. [Crossref] [PubMed]
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators, Cavalcanti AB, Suzumura EA, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017;318:1335-45. [Crossref] [PubMed]
- Fish E, Novack V, Banner-Goodspeed VM, et al. The Esophageal Pressure-Guided Ventilation 2 (EPVent2) trial protocol: a multicentre, randomised clinical trial of mechanical ventilation guided by transpulmonary pressure. BMJ Open 2014;4. [Crossref] [PubMed]
- Loring SH, O'Donnell CR, Behazin N, et al. Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol (1985) 2010;108:515-22. [Crossref] [PubMed]
- Loring SH, Pecchiari M, Della Valle P, et al. Maintaining end-expiratory transpulmonary pressure prevents worsening of ventilator-induced lung injury caused by chest wall constriction in surfactant-depleted rats. Crit Care Med 2010;38:2358-64. [Crossref] [PubMed]
- Baedorf Kassis E, Loring SH, Talmor D. Esophageal pressure: research or clinical tool? Med Klin Intensivmed Notfmed 2018;113:13-20. [Crossref] [PubMed]
- Mauri T, Yoshida T, Bellani G, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med 2016;42:1360-73. [Crossref] [PubMed]
- Akoumianaki E, Maggiore SM, Valenza F, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014;189:520-31. [Crossref] [PubMed]
- Brochard L. Measurement of esophageal pressure at bedside: pros and cons. Curr Opin Crit Care 2014;20:39-46. [Crossref] [PubMed]
- Gattinoni L, Carlesso E, Cadringher P, et al. Physical and biological triggers of ventilator-induced lung injury and its prevention. Eur Respir J Suppl 2003;47:15s-25s. [Crossref] [PubMed]
- Gattinoni L, Carlesso E, Caironi P. Stress and strain within the lung. Curr Opin Crit Care 2012;18:42-7. [Crossref] [PubMed]
- Chiumello D, Cressoni M, Colombo A, et al. The assessment of transpulmonary pressure in mechanically ventilated ARDS patients. Intensive Care Med 2014;40:1670-8. [Crossref] [PubMed]
- Chiumello D, Guerin C. Understanding the setting of PEEP from esophageal pressure in patients with ARDS. Intensive Care Med 2015;41:1465-7. [Crossref] [PubMed]
- Chiumello D, Carlesso E, Cadringher P, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med 2008;178:346-55. [Crossref] [PubMed]
- Gulati G, Novero A, Loring SH, et al. Pleural pressure and optimal positive end-expiratory pressure based on esophageal pressure versus chest wall elastance: incompatible results*. Crit Care Med 2013;41:1951-7. [Crossref] [PubMed]
- Loring SH, Topulos GP, Hubmayr RD. Transpulmonary Pressure: The Importance of Precise Definitions and Limiting Assumptions. Am J Respir Crit Care Med 2016;194:1452-7. [Crossref] [PubMed]
- Gattinoni L, Cressoni M, Chiumello D, et al. Transpulmonary Pressure Meaning: Babel or Conceptual Evolution? Am J Respir Crit Care Med 2017;195:1404-5. [Crossref] [PubMed]
- Loring SH, Topulos GP, Hubmayr RD. Reply: Transpulmonary Pressure Meaning: Babel or Conceptual Evolution? Am J Respir Crit Care Med 2017;195:1405-6. [Crossref] [PubMed]
- Cherniack RM, Farhi LE, Armstrong BW, et al. A comparison of esophageal and intrapleural pressure in man. J Appl Physiol 1955;8:203-11. [Crossref] [PubMed]
- Yoshida T, Amato MBP, Grieco DL, et al. Esophageal Manometry and Regional Transpulmonary Pressure in Lung Injury. Am J Respir Crit Care Med 2018;197:1018-26. [Crossref] [PubMed]
- Washko GR, O'Donnell CR, Loring SH. Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J Appl Physiol (1985) 2006;100:753-8. [Crossref] [PubMed]
- Pelosi P, Goldner M, McKibben A, et al. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 2001;164:122-30. [Crossref] [PubMed]
- Pecchiari M, Loring SH, D'Angelo E. Esophageal pressure as an estimate of average pleural pressure with lung or chest distortion in rats. Respir Physiol Neurobiol 2013;186:229-35. [Crossref] [PubMed]
- Behazin N, Jones SB, Cohen RI, et al. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol (1985) 2010;108:212-8. [Crossref] [PubMed]
- Fumagalli J, Berra L, Zhang C, et al. Transpulmonary Pressure Describes Lung Morphology During Decremental Positive End-Expiratory Pressure Trials in Obesity. Crit Care Med 2017;45:1374-81. [Crossref] [PubMed]
- Gillespie DJ, Lai YL, Hyatt RE. Comparison of esophageal and pleural pressures in the anesthetized dog. J Appl Physiol 1973;35:709-13. [Crossref] [PubMed]
- Terragni P, Mascia L, Fanelli V, et al. Accuracy of esophageal pressure to assess transpulmonary pressure during mechanical ventilation. Intensive Care Med 2017;43:142-3. [Crossref] [PubMed]
- Baedorf Kassis E, Loring SH, Talmor D, et al. A fixed correction of absolute transpulmonary pressure may not be ideal for clinical use: Discussion on "Accuracy of esophageal pressure to assess transpulmonary pressure during mechanical ventilation". Intensive Care Med 2017;43:1436-7. [Crossref] [PubMed]
- Mojoli F, Iotti GA, Torriglia F, et al. In vivo calibration of esophageal pressure in the mechanically ventilated patient makes measurements reliable. Crit Care 2016;20:98. [Crossref] [PubMed]
- Chiumello D, Consonni D, Coppola S, et al. The occlusion tests and end-expiratory esophageal pressure: measurements and comparison in controlled and assisted ventilation. Ann Intensive Care 2016;6:13. [Crossref] [PubMed]
- Norisue Y, Ashworth L, Naito T, et al. Impact of physician education and availability of parameters regarding esophageal pressure and transpulmonary pressure on clinical decisions involving ventilator management. J Crit Care 2017;41:112-8. [Crossref] [PubMed]
- Malbrain ML, Chiumello D, Pelosi P, et al. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med 2004;30:822-9. [Crossref] [PubMed]
- Bikker IG, Blankman P, Specht P, et al. Global and regional parameters to visualize the 'best' PEEP during a PEEP trial in a porcine model with and without acute lung injury. Minerva Anestesiol 2013;79:983-92. [PubMed]
- Pirrone M, Fisher D, Chipman D, et al. Recruitment Maneuvers and Positive End-Expiratory Pressure Titration in Morbidly Obese ICU Patients. Crit Care Med 2016;44:300-7. [Crossref] [PubMed]
- Zhang C, Pirrone M, Imber DA, et al. Optimization of Mechanical Ventilation in a 31-Year-Old Morbidly Obese Man With Refractory Hypoxemia. A A Case Rep 2017;8:7-10. [Crossref] [PubMed]
- Grasso S, Terragni P, Birocco A, et al. ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med 2012;38:395-403. [Crossref] [PubMed]
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Loring SH, Malhotra A. Driving pressure and respiratory mechanics in ARDS. N Engl J Med 2015;372:776-7. [Crossref] [PubMed]
- Baedorf Kassis E, Loring SH, Talmor D. Mortality and pulmonary mechanics in relation to respiratory system and transpulmonary driving pressures in ARDS. Intensive Care Med 2016;42:1206-13. [Crossref] [PubMed]
- Protti A, Cressoni M, Santini A, et al. Lung stress and strain during mechanical ventilation: any safe threshold? Am J Respir Crit Care Med 2011;183:1354-62. [Crossref] [PubMed]
- Cressoni M, Cadringher P, Chiurazzi C, et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2014;189:149-58. [PubMed]
- Hess DR. Respiratory mechanics in mechanically ventilated patients. Respir Care 2014;59:1773-94. [Crossref] [PubMed]


