Fibroblast growth factors 19 and 21 in acute liver damage
Introduction
The fibroblast growth factor (FGF) family comprises twenty two members in human and mice, which are classified in six subfamilies according to their structural characteristics and mechanisms of action (1,2). Besides having a key role in embryonic development these polypeptides are involved in a wide variety of biological actions including the regulation of cell growth, differentiation, wound healing, angiogenesis and metabolism (2,3). FGFs signal through four different tyrosine kinase receptors termed FGFR1 to 4, and this interaction is markedly strengthened by heparin or heparan sulphate glycosaminoglycans (4,5). Importantly, depending on the tissue type different splicing forms based on the alterative incorporation of exons IIIb and IIIc of the genes coding for FGFRs can be found. Therefore a total of seven different isoforms of FGFRs have been characterized, namely FGFR1b, FGFR1c, FGFR2b, FGFR2c, FGFR3b, FGFR3c and FGFR4 (6). FGFR binding leads to activation, dimerization and the triggering of intracellular signaling pathways (7). Most of the FGFs interact with heparin moieties and behave as autocrine and paracrine factors, however the members of the FGF19 subfamily of FGFs (FGF19, FGF21 and FGF23) lack a classic heparin binding domain (8). This feature facilitates the diffusion of these proteins from the tissue of production and their secretion into the bloodstream, thus allowing an endocrine mode of action (9). However, lack of heparin binding domain in the FGF19 subfamily is accompanied by a low affinity interaction with the FGFRs. This situation is compensated by the presence of a transmembrane co-receptor named Klotho, which dimerizes with and contributes to activate the FGFRs (3,10). There are two major Klotho proteins, α-Klotho and β-Klotho, and their tissue-specific pattern of expression restricts to a great extent the target organs on which endocrine FGFs exert their biological activities (11-15).
FGF19, FGF21 and FGF23 are increasingly recognized as important hormones in the systemic regulation of metabolism. The central metabolic pathways controlled by these factors include carbohydrate, lipid and bile acid metabolism, as well as vitamin D and phosphate homeostasis (3,10,16,17). Alterations in the levels of the endocrine FGFs have been described in different pathological conditions, including chronic diseases such as obesity and type 2 diabetes, as well as devastating pathologies like liver cancer and bone diseases (3). Therefore, pharmaceutical strategies aimed at stimulating or inhibiting FGFs signaling are actively being pursued (3,18,19). However, the chronic stimulation, or repression, of the metabolic pathways controlled by these hormones may involve important challenges, including the risk of neoplastic transformation due to the mitogenic potential of factors like FGF19 (18). Therefore, many efforts are being dedicated to design FGF-based molecules with improved physical or pharmacokinetic properties, such as the FGF21-related molecules LY2405319 and CVX-343 (20,21), or the NGM282 FGF19 variant devoid of mitogenic effects (22). The potential application of FGF19, FGF21, and their engineered versions, to treat chronic metabolic conditions has been recently reviewed (3,23). However, the liver, which is a major direct or indirect target organ for FGF19 and FGF21, can also undergo acute episodes of injury and dysfunction which may have fatal consequences. In this review, we briefly revisit the biology of FGF19 and FGF21, outline the several major causes and clinical problems of acute liver injury, and discuss the potential therapeutic applications of FGF19 and FGF21 in acute hepatic damage of selected aetiologies.
Overview of FGF19 and FGF21 biology
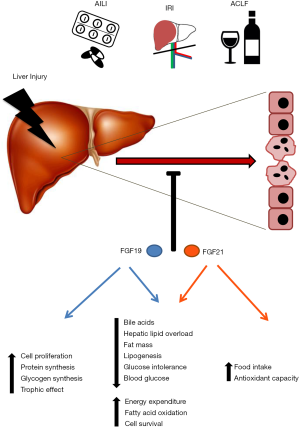
FGF19 was cloned by homology to the mouse orthologue Fgf15 from fetal brain tissues and retina (24). FGF19 and Fgf15, expression is detected mainly in the small intestine, gallbladder, brain, cartilage, skin and kidney (6,25). The fundamental source of endocrine FGF19 is the ileum, from where it is released into the portal circulation. The expression of FGF19 in enterocytes is triggered by bile acids in their enterohepatic circulation through multiple farnesoid X receptor (FXR) binding sites in the FGF19 gene (26,27). This mechanism makes FGF19 a postprandial hormone, peaking in human serum approximately 2–3 h after a meal (28). More recently, additional studies demonstrated the induction of FGF19/Fgf15 ileal expression by vitamins A and D (29,30) and other nutrients such as carbohydrates (31) and cholesterol (32). Interestingly, the FGF19/Fgf15 promoter in mouse ileal enterocytes is also activated by endoplasmic reticulum (ER) stress caused by non-physiological ER stress triggering molecules (33), but also by nutrients like saturated fatty acids at high concentrations (34). Importantly, under excessive accumulation of intrahepatic bile acids FGF19/Fgf15 expression was observed in human but not in murine liver (34-36). Once in the circulation, FGF19 can interact with its target organs and tissues, which are those expressing FGFR4 and/or FGFR1c together with β-Klotho. Both, FGFR4 and β-Klotho are highly expressed in hepatocytes, where FGF19 has strong effects on the suppression of bile acid synthesis, gluconeogenesis and fatty acid synthesis, while it induces protein and glycogen synthesis (14,16,34,37). The adipose tissue, both white and brown, is also a target of FGF19 as it expresses high levels of FGFR1c together with β-Klotho. FGF19 effects on adipose tissue are believed to be important for glucose and lipid homeostasis (10,16). Finally, recent evidence also points to glucose lowering effects of FGF19 via the central nervous system through still not fully characterized mechanisms (38).
Pharmacological administration, or transgenic overexpression, of FGF19 in mice confirmed the strong physiological effects of this hormone in reducing liver fat accumulation and bile acid synthesis (22,36,39,40). Intriguingly, a recent report demonstrated that the long term pharmacological effects of FGF19 on weight loss and glycemia were mainly mediated at the level of the nervous system (41). Similar pharmacological approaches also evidenced a potent trophic effect for FGF19 in skeletal muscle (42) and hepatocytes (43). Importantly, the persistent activation of FGFR4 by FGF19 on hepatocytes leads to active proliferation and the eventual development of hepatocellular carcinoma (44). Binding of FGFR4/β-Klotho activates a growing number of downstream intracellular signaling pathways, including the Ras-Raf-Erk1/2 mitogen-activated protein kinase and phosphoinositide 3-kinase pathways, the Jun N-terminal kinase pathway, the glycogen synthase kinase 3β (GSK3β)-β-catenin pathway and the mechanistic target of rapamycin 1 (mTORC1) pathway, among others. These signaling systems are responsible of the metabolic, proliferative and trophic effects of FGF19 in hepatocytes (18,45).
Human and murine FGF21 were cloned from mouse embryo and fetal brain cDNA libraries respectively (46). In mice, it is predominantly expressed in the liver and adipose tissues, and at much lower levels in other organs like heart, kidney and skeletal muscle (6,47). In humans, under basal conditions, FGF21 expression is found almost exclusively in the liver (47). Hepatic expression of Fgf21 is strongly induced in the mouse liver by prolonged fasting. The peroxisome proliferator activated receptor α (PPARα), which is activated by increased circulating free fatty acids, is necessary for Fgf21 upregulation upon fasting and high-fat low carbohydrate ketogenic diets (16,47). High sugar ingestion also triggers hepatic FGF21 expression in mice and human, the effect of fructose being particularly strong (48). Other hormones, such as glucocorticoids, also induce Fgf21 expression in the liver (47,49). Interestingly, stress situations such as amino acid deprivation and protein restriction result in robust FGF21 hepatic production through the ATF4-CHOP axis of the ER stress response pathway in mice (50,51). The notion of FGF21 as a hormone produced by the liver under stress conditions has been substantiated in several models of liver inflammation, liver injury elicited by ethanol, drugs or ischemia/reperfusion (IR), liver regeneration and hepatocarcinogenesis (52-55).
FGF21 exerts potent effects on systemic glucose and lipid metabolism. FGF21 administration to obese mice reduces body weight, fat mass and hepatic triglyceride content, improving glucose tolerance and insulin sensitivity, as reviewed in (3,16,47). Treatment with FGF21 stimulates hepatic fatty acid oxidation and suppresses de novo lipogenesis, while FGF21 deficient mice show hepatic insulin resistance and increased glucose production (47). These outcomes are mediated through FGF21 interaction with the FGFR1c receptor in combination with β-Klotho (3). Of note, experimental evidence identified the white and brown adipose tissues as major targets for the metabolic effects of this hormone (56,57), while direct action on liver parenchymal cells could never be observed (58). In fact, while some effects of FGF21 on hepatic metabolism, such as that of cholesterol, might be mediated through FGFR2/β-Klotho signaling in hepatocytes (59), most of the effects of this hormone on liver cells are believed to occur indirectly. One important mediator of FGF21 actions in the liver is the adipocyte-derived hormone adiponectin, which is strongly upregulated by FGF21 (56). Adiponectin-null mice fed a high fat diet are refractory to the metabolic effects of FGF21, including the attenuation of hepatosteatosis (60). Interestingly, a recent study using tissue-specific β-Klotho knockout mice demonstrated that besides adipose tissue the pharmacological long term effects of FGF21 on glucose metabolism were mediated at the level of the nervous system (41).
Acute liver damage: causes and available therapeutic options
Excessive liver injury may lead to acute liver failure (ALF), a rare but life-threatening condition, characterized by a rapid loss of liver function along with coagulopathy and encephalopathy. The incidence of ALF in the US is approximately 2,000 cases per year, accounting for about 6% of all liver injury-related deaths (61). ALF can occur in young adults who have no pre-existing liver disease and thus presents a significant clinical challenge. The effective treatment of ALF remains to be liver transplantation even though advances in critical care management to alleviate symptoms have largely increased the survival rate of ALF patients in recent years. There are a multitude of causes for ALF. Drug-induced liver injury accounts for the majority of ALF cases in developed world (62). In addition, hepatic I/R injury can cause ALF in patients undergoing trauma or liver surgery (63-66). Here, we outline the several major causes and clinical problems of ALF and in the next section we discuss the potential therapeutic application of FGF19/FGF21 in ALF patients.
Acetaminophen (APAP)-induced liver injury (AILI) is the most common cause of ALF in the United States. APAP is the active component in many prescribed and over-the-counter medications commonly used to treat fever and pain (67). Although the hepatotoxicity caused by APAP overdose was discovered over 50 years ago, it was not until 2014 when the US Food and Drug Administration issued a guideline to limit its consumption to less than 4 g per day. This dose is usually safe, but people under certain situations (e.g., alcohol abuse, chronic liver disease, malnutrition, aged) may have lower tolerance to APAP and develop acute liver injury under lower doses (68). A study of 275 patients who developed AILI due to APAP overdose revealed that there were 48% un-intentional overdoes, 44% intentional and 8% of unknown intent (69). In this cohort, 65% survived, 27% died without transplantation and 8% underwent transplantation (69). Another report estimated that 60 million Americans take APAP-containing products weekly and approximately 30,000 patients are admitted to intensive care units every year due to AILI (63,70). The direct cost of APAP overdose-induced liver injury has been estimated to be as high as US $87 million annually (71).
APAP can cause centrilobular hepatic necrosis through bioactivation to form a reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which depletes glutathione (GSH), binds to mitochondrial proteins, increases oxidative stress and eventually leads to mitochondrial dysfunction and cell necrosis. Once necrosis occurs, the release of damage-associated molecular pattern molecules (DAMPs) induces an inflammatory response that further exacerbates liver injury (72). The timing of hospitalization after APAP overdose is critical for their survival. Gastric lavage, activated charcoal ingestion and ipecacuanha-induced vomiting within 4 h of APAP ingestion are proven effective in attenuating drug absorption (73). N-acetylcysteine (NAC), the GSH precursor, can replenish GSH stores and thus detoxify NAPQI (74). However, NAC can prevent hepatic injury only if given within 12 h post APAP exposure. Unfortunately, unintentional overdosing is not often recognized until later (69). If AILI develops into fulminant liver failure, liver transplantation represents the only life-saving procedure. Due to the shortage of donor organs, only a small fraction of patients with APAP poisoning receive transplantation (75). Therefore, it is imperative to explore new antidote strategies, perhaps those that promote liver regeneration.
Liver I/R injury (IRI) is another major cause of acute liver injury. It results from acute cessation of blood supply to liver followed by restoration of blood flow, the latter causing significant cellular injury and subsequent liver dysfunction (76). There are two types of liver IRI: warm and cold. Warm I/R can occur during hemorrhagic shock, trauma, liver surgery and transplantation. If the ischemia time is longer than 15 minutes during warm I/R, reperfusion will greatly contribute to high morbidities (77). Hepatic IRI represents a key risk factor in postoperative recovery (78). Cold I/R occurs during organ preservation. Two-fold increase of liver IRI has been consistently seen if cold preservation of liver is longer than 14 h in vitro (79). In the US, there is a significant shortage of donor organs, resulting in approximately 15% yearly mortality of patients waiting for liver transplantation (80). To deal with this problem, marginal organs collected from older, steatotic, or non-heart-beating donors, have been used for liver transplantation. Unfortunately, these marginal organs are more prone to IRI (79). At present, treatment options for liver IRI are extremely limited (81,82). Approaches to reduce liver IRI could improve postoperative/transplant outcomes and help save organs that would be disposed otherwise.
Acute-on-chronic liver failure (ACLF) is recognized as an acute deterioration of liver function in patients with pre-existing chronic liver disease (83). ACLF can be classified into three types. Type A is defined as non-cirrhotic ACLF. However, type B and C occur in approximately 31% of hospitalized patients with liver cirrhosis (84). Type B is defined as compensated cirrhosis with an acute hepatic deterioration caused by infection, surgery or acute alcoholic hepatitis. Type C is decompensated cirrhosis with an acute hepatic deterioration caused by similar etiologies as in type B. The most common cause of ACLF is alcoholic liver disease, accompanied by infection and kidney failure (85). Therapy for severe alcoholic steatohepatitis is currently limited to corticosteroids administration, with an improved effect when given in combination with NAC (86).
ACLF is often associated with failure of one or more organs and has a high short-term mortality (84,87). A clinical survey of 223 patients who were diagnosed with ACLF revealed a 90-day transplant-free mortality rate as high as 62% (84). Clinical managements during early days of hospital stay significantly affect the survival outcome (84). Various devices that support the liver have been explored. Although ineffective in improving overall survival, they could stabilize liver function in certain patients until transplantation (88). Due to the rapid progression of ACLF, a larger proportion of patients are either not eligible for transplantation or die before transplantation. A meta-analysis of 303 patients with ACLF demonstrated that only 15% underwent transplantation within 90 days (89). A multicentered study from Canada showed that 48% of patients died while waiting for liver transplantation (90). In order to improve survival, introduction of effective interventions to prevent liver deterioration, improve liver function and foster hepatocyte survival and regeneration will be particularly important.
Potential hepatoprotective application of FGF19 and FGF21 in acute liver damage
The biological activities of FGF19 and FGF21 summarized above suggested that these hormones could have a beneficial effect on liver injury, including acute forms of liver damage. Regarding FGF19, early evidence of hepatoprotective potential was obtained in mice that were subjected to acute injury induced upon ligation of the common bile duct (BDL). In these animals, injection of FGF19 markedly reduced the total bile acid pool size and the extent of extrahepatic cholestasis-induced liver necrosis (91). Protection from cholestasis-associated liver injury by recombinant FGF19 administration was confirmed in a model of chemically-induced biliary epithelial cell damage (22). As previously mentioned, FGF19 has strong mitogenic effects in hepatocytes, an effect that may lead to hepatocellular carcinoma development in prolonged treatments (18,92). To avoid this, non-tumorigenic variants of FGF19 have been developed, and these molecules have shown hepatoprotective effects in chronic liver injury associated with alcoholic and non-alcoholic steatohepatitis and cholestasis through the modulation of lipid and bile acid metabolism (93,94). However, the combined metabolic and mitogenic effects of FGF19/FGF15 may be important in clinically relevant situations where liver regeneration is needed. A first evidence in this regard was obtained in a model of ALF due to extensive parenchymal resection (85% partial hepatectomy, PH), where administration of FGF15 expressed from an adenoviral vector markedly improved mouse survival (36). In the clinic, the presence of cholestasis often associated with steatosis existing prior to liver resection, or developing after transplantation, has a negative impact on liver regeneration (95-98). In this context, pre-operative administration of FGF19 from adeno-associated viral vectors to obese db/db mice with fatty liver improved survival after extensive PH (85%). While these effects confirm the efficacy of a FGF19-based therapy, from a translational point of view the use of recombinant factors instead of viral vectors is preferred. In the case of FGF19, one limitation to its clinical application is the short half-life of the protein (3,22). To overcome this limitation, a chimaeric molecule based on the fusion of FGF19 with apolipoprotein A-I (ApoA-I) has been synthesized. This molecule, named Fibapo, demonstrated not only an extended half-life, but also increased hepatotropism owing to the interaction of the ApoA-I moiety with the scavenger receptor class B type I (SR-BI) highly expressed in hepatocytes (99). Pre-operative administration of Fibapo to obese (db/db) mice markedly improved survival and liver regeneration after 70% PH (34). Mechanistically, this effect could be attributed to a marked reduction of liver steatosis and a strong hepatotrophic effect, factors that avoid lipotoxicity and stimulate liver growth, therefore enhancing liver function in the critical hours after parenchymal resection (97,100-102). Similarly, administration of Fibapo prior to PH significantly reduced liver injury and improved regeneration in aged mice (103), in which liver regrowth after partial resection is impaired, as occurs in elderly patients (104). This protective and pro-regenerative effect could be also related to the improvement of the steatosis commonly present in aged livers, as well as to the strong trophic and mitogenic effects elicited by Fibapo (103). Interestingly, delayed administration of Fibapo also protected from APAP-induced liver injury and increased mice survival after lethal doses of the drug, performing better than NAC (103). Mechanistically, the activation of pro-survival and cell growth-related intracellular signaling pathways, along with the inhibition of the pro-apoptotic mitochondria-associated phospho-JNK (p-JNK) (105,106), could be responsible for the beneficial effects of Fibapo on APAP-induced liver injury (103). In view of these findings, it could be interesting to evaluate the hepatoprotective effects of FGF19, or related molecules such as Fibapo, on other models of acute liver injury like acute ethanol intoxication or I/R mediated liver damage.
There are also some recent lines of evidence on the hepatoprotective capacity of FGF21. As mentioned before, FGF21 expression in the liver is increased upon cell stress and injury elicited by different agents, including APAP (55). Indeed, APAP administration results in the fast and strong upregulation of hepatic FGF21 expression and secretion into the circulation (55). Interestingly, APAP-mediated increase in FGF21 expression was independent of PPARα, a major regulator of Fgf21 gene expression in hepatocytes (47). Of note, in the absence of FGF21, i.e., FGF21 null mice, APAP hepatotoxicity and mortality was exacerbated, and recombinant FGF21 administration had significant protective effects (55). Moreover, a recent study showed that the beneficial effects of glucocorticoid pretreatment on APAP-induced liver injury required FGF21 expression (107). Regarding the mechanisms responsible for FGF21 hepatoprotection, FGF21 null mice showed marked hepatic oxidative stress upon APAP intoxication, along with increased mitochondrial p-JNK levels, and treatment with recombinant FGF21 restored liver antioxidant activity (55). This response was attributed to the activation of the transcriptional coactivator peroxisome proliferator-activated receptor coactivator protein 1α (PGC1α) by FGF21 administration (55). PGC1α controls the expression of a variety of antioxidant genes, including that of the nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of antioxidant gene expression (108). Importantly, hepatic Nrf2 expression, which is quickly activated upon APAP administration, was markedly impaired in FGF21 null mice (55). Another study also demonstrated a protective effect of FGF21 on D-galactose-induced mouse liver injury, and this was also related to the activation of Nrf2-mediated antioxidant capacity (109). One key unanswered issue in FGF21-mediated hepatoprotection, as well as in FGF21-mediated hepatic metabolic regulation (41,58), is how this hormone exerts its effects on the liver parenchyma. Indeed, treatment of isolated hepatocytes with FGF21 had no effect on PGC1α nor Nrf2 expression (55,58), therefore an indirect mechanism involving the activation of hepatoprotective factor/s by FGF21 must exist.
There is also evidence indicating that FGF21 can ameliorate liver injury caused by chronic alcohol consumption. Indeed, FGF21 null mice develop increased hepatic damage, including inflammation, steatosis and fibrosis, and show higher mortality than their wild type counterparts when fed an ethanol supplemented diet (54). Interestingly, acute ethanol administration markedly increases FGF21 serum levels in mice and humans, and Fgf21 gene expression in mice (54). On the other hand, the relationship between Nrf2 and FGF21 seems to be reciprocal. Besides the above-mentioned stimulatory effect of this hormone on Nrf2 expression, one study found that the induction of Fgf21 expression by chronic ethanol feeding was attenuated in Nrf2 null mice, which as expected display increased liver injury when fed an ethanol supplemented diet (110). These findings suggest that the hepatic upregulation of FGF21 expression upon APAP intoxication discussed above could also be mediated in part through Nrf2 activation.
Different engineered forms of FGF21 have been generated with the aim of avoiding protein aggregation, increase conformational stability, avoiding proteolysis or increasing half-life [reviewed in (3)]. The pharmacological activity of some of these variants, such as LY2405319 and PF05231023, has been assessed in experimental models of NAFLD and in patients with obesity and type 2 diabetes, with promising results (3,111,112). It would be very interesting to test the effects of these improved FGF21 variants in experimental models of liver injury elicited by I/R, APAP overdose, acute ethanol intoxication and ACLF.
Conclusions
There are currently very few pharmacological options available to prevent or treat acute liver injury. One potential source of hepatoprotective agents may reside in the endogenous reparative response elicited both locally and systemically upon liver injury. The administration of these agents may enhance the natural regenerative responses of the organism. Moreover, the biological activities of these protective factors can be harnessed in semisynthetic derivatives with improved kinetic and pharmacological properties. In this review, we have summarized evidence suggesting that this could be the case for the endocrine fibroblast growth factors FGF19 and FGF21 (Figure 1).
Acknowledgements
Funding: This work was supported by Ministerio de Economía (Mineco), Spain (grant numbers SAF 2016-75972R, SAF2014-54191R and SAF 2017-88933R), Ministerio de Sanidad, Instituto Carlos III, Spain (grant number FIS PI16/01126) and the National Institutes of Health USA (U01AA021723, R21AA024636, R01DK109574). Marie Curie EU contract to MGF-B. Fundación La Caixa Hepacare Project; Fundación Eugenio Rodríguez Pascual; Fundación M. Torres; Fundación Fuentes Dutor; Fundación Mario Losantos; Fundación Familia Puig-Infante; BiO-Eusko Fundazioa, Spain (grant number BIO15/CA/011). The generous support of Mr. Eduardo Avila and Mr. Sergio Durá is highly appreciated
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends in Genetics 2004;20:563-9. [Crossref] [PubMed]
- Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 2009;8:235-53. [Crossref] [PubMed]
- Degirolamo C, Sabbà C, Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov 2016;15:51-69. [Crossref] [PubMed]
- Ornitz DM. FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays 2000;22:108-12. [Crossref] [PubMed]
- Zhang X, Ibrahimi OA, Olsen SK, et al. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem 2006;281:15694-700. [Crossref] [PubMed]
- Fon Tacer K, Bookout AL, Ding X, et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol 2010;24:2050-64. [Crossref] [PubMed]
- Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev 2005;16:107-37. [Crossref] [PubMed]
- Goetz R, Beenken A, Ibrahimi OA, et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol 2007;27:3417-28. [Crossref] [PubMed]
- Itoh N. Hormone-like (endocrine) Fgfs: their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res 2010;342:1-11. [Crossref] [PubMed]
- Zhang F, Yu L, Lin X, et al. Minireview: Roles of Fibroblast Growth Factors 19 and 21 in Metabolic Regulation and Chronic Diseases. Mol Endocrinol 2015;29:1400-13. [Crossref] [PubMed]
- Kurosu H, Choi M, Ogawa Y, et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 2007;282:26687-95. [Crossref] [PubMed]
- Lin BC, Wang M, Blackmore C, et al. Liver-specific activities of FGF19 require Klotho beta. J Biol Chem 2007;282:27277-84. [Crossref] [PubMed]
- Ding X, Boney-Montoya J, Owen BM, et al. βKlotho Is Required for Fibroblast Growth Factor 21 Effects on Growth and Metabolism. Cell Metabolism 2012;16:387-93. [Crossref] [PubMed]
- Owen BM, Mangelsdorf DJ, Kliewer SA. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol Metab 2015;26:22-9. [Crossref] [PubMed]
- Itoh N, Nakayama Y, Konishi M. Roles of FGFs As Paracrine or Endocrine Signals in Liver Development, Health, and Disease. Front Cell Dev Biol 2016;4:30. [PubMed]
- Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev 2012;26:312-24. [Crossref] [PubMed]
- Pool LR, Wolf M. FGF23 and Nutritional Metabolism. Annu Rev Nutr 2017;37:247-68. [Crossref] [PubMed]
- Alvarez-Sola G, Uriarte I, Latasa MU, et al. Fibroblast Growth Factor 15/19 in Hepatocarcinogenesis. Dig Dis 2017;35:158-65. [Crossref] [PubMed]
- Fukumoto S. Targeting Fibroblast Growth Factor 23 Signaling with Antibodies and Inhibitors, Is There a Rationale? Front Endocrinol (Lausanne) 2018;9:48. [Crossref] [PubMed]
- Kharitonenkov A, Beals JM, Micanovic R, et al. Rational design of a fibroblast growth factor 21-based clinical candidate, LY2405319. PLoS One 2013;8. [Crossref] [PubMed]
- Huang J, Ishino T, Chen G, et al. Development of a novel long-acting antidiabetic FGF21 mimetic by targeted conjugation to a scaffold antibody. J Pharmacol Exp Ther 2013;346:270-80. [Crossref] [PubMed]
- Luo J, Ko B, Elliott M, et al. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med 2014;6. [Crossref] [PubMed]
- Izaguirre M, Gil MJ, Monreal I, et al. The Role and Potential Therapeutic Implications of the Fibroblast Growth Factors in Energy Balance and Type 2 Diabetes Curr Diab Rep 2017;17:43. [Crossref] [PubMed]
- Xie MH, Holcomb I, Deuel B, et al. FGF-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine 1999;11:729-35. [Crossref] [PubMed]
- Nishimura T, Utsunomiya Y, Hoshikawa M, et al. Structure and expression of a novel human FGF, FGF-19, expressed in the fetal brain. Biochim Biophys Acta 1999;1444:148-51. [Crossref] [PubMed]
- Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metabolism 2005;2:217-25. [Crossref] [PubMed]
- Miyata M, Hata T, Yamakawa H, et al. Involvement of multiple elements in FXR-mediated transcriptional activation of FGF19. J Steroid Biochem Mol Biol 2012;132:41-7. [Crossref] [PubMed]
- Lundåsen T, Gälman C, Angelin B, et al. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med 2006;260:530-6. [Crossref] [PubMed]
- Schmidt DR, Holmstrom SR, Fon Tacer K, et al. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J Biol Chem 2010;285:14486-94. [Crossref] [PubMed]
- Jahn D, Sutor D, Dorbath D, et al. Farnesoid X receptor-dependent and -independent pathways mediate the transcriptional control of human fibroblast growth factor 19 by vitamin A. Biochim Biophys Acta 2016;1859:381-92. [Crossref] [PubMed]
- Morton GJ, Kaiyala KJ, Foster-Schubert KE, et al. Carbohydrate feeding dissociates the postprandial FGF19 response from circulating bile acid levels in humans. J Clin Endocrinol Metab 2014;99:E241-5. [Crossref] [PubMed]
- Henkel AS, Anderson KA, Dewey AM, et al. A chronic high-cholesterol diet paradoxically suppresses hepatic CYP7A1 expression in FVB/NJ mice. J Lipid Res 2011;52:289-98. [Crossref] [PubMed]
- Shimizu M, Li J, Maruyama R, et al. FGF19 (fibroblast growth factor 19) as a novel target gene for activating transcription factor 4 in response to endoplasmic reticulum stress. Biochem J 2013;450:221-9. [Crossref] [PubMed]
- Alvarez-Sola G, Uriarte I, Latasa MU, et al. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis: development of an FGF19-based chimeric molecule to promote fatty liver regeneration. Gut 2017;66:1818-28. [Crossref] [PubMed]
- Schaap FG, van der Gaag NA, Gouma DJ, et al. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 2009;49:1228-35. [Crossref] [PubMed]
- Uriarte I, Fernandez-Barrena MG, Monte MJ, et al. Identification of fibroblast growth factor 15 as a novel mediator of liver regeneration and its application in the prevention of post-resection liver failure in mice. Gut 2013;62:899-910. [Crossref] [PubMed]
- Kir S, Kliewer SA, Mangelsdorf DJ. Roles of FGF19 in liver metabolism. Cold Spring Harb Symp Quant Biol 2011;76:139-44. [Crossref] [PubMed]
- Morton GJ, Matsen ME, Bracy DP, et al. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest 2013;123:4799-808. [Crossref] [PubMed]
- Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 2004;145:2594-603. [Crossref] [PubMed]
- Tomlinson E, Fu L, John L, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 2002;143:1741-7. [Crossref] [PubMed]
- Lan T, Morgan DA, Rahmouni K, et al. FGF19, FGF21, and an FGFR1/β-Klotho-Activating Antibody Act on the Nervous System to Regulate Body Weight and Glycemia. Cell Metabolism 2017;26:709-18.e3. [Crossref] [PubMed]
- Benoit B, Meugnier E, Castelli M, et al. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat Med 2017;23:990-6. [PubMed]
- Nicholes K, Guillet S, Tomlinson E, et al. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am J Pathol 2002;160:2295-307. [Crossref] [PubMed]
- French DM, Lin BC, Wang M, et al. Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS One 2012;7. [Crossref] [PubMed]
- Jahn D, Rau M, Hermanns HM, et al. Mechanisms of enterohepatic fibroblast growth factor 15/19 signaling in health and disease. Cytokine Growth Factor Rev 2015;26:625-35. [Crossref] [PubMed]
- Nishimura T, Nakatake Y, Konishi M, et al. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 2000;1492:203-6. [Crossref] [PubMed]
- Staiger H, Keuper M, Berti L, et al. Fibroblast Growth Factor 21-Metabolic Role in Mice and Men. Endocr Rev 2017;38:468-88. [Crossref] [PubMed]
- Lundsgaard AM, Fritzen AM, Sjøberg KA, et al. Circulating FGF21 in humans is potently induced by short term overfeeding of carbohydrates. Mol Metab 2016;6:22-9. [Crossref] [PubMed]
- Patel R, Bookout AL, Magomedova L, et al. Glucocorticoids regulate the metabolic hormone FGF21 in a feed-forward loop. Mol Endocrinol 2015;29:213-23. [Crossref] [PubMed]
- Laeger T, Henagan TM, Albarado DC, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest 2014;124:3913-22. [Crossref] [PubMed]
- De Sousa-Coelho AL, Marrero PF, Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochem J 2012;443:165-71. [Crossref] [PubMed]
- Yang C, Lu W, Lin T, et al. Activation of Liver FGF21 in hepatocarcinogenesis and during hepatic stress. BMC Gastroenterol 2013;13:67. [Crossref] [PubMed]
- Ye D, Li H, Wang Y, et al. Circulating Fibroblast Growth Factor 21 Is A Sensitive Biomarker for Severe Ischemia/reperfusion Injury in Patients with Liver Transplantation. Sci Rep 2016;6:19776. [Crossref] [PubMed]
- Desai BN, Singhal G, Watanabe M, et al. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol Metab 2017;6:1395-406. [Crossref] [PubMed]
- Ye D, Wang Y, Li H, et al. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1α-mediated antioxidant capacity in mice. Hepatology 2014;60:977-89. [Crossref] [PubMed]
- Adams AC, Yang C, Coskun T, et al. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Mol Metab 2012;2:31-7. [Crossref] [PubMed]
- Emanuelli B, Vienberg SG, Smyth G, et al. Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest 2014;124:515-27. [Crossref] [PubMed]
- Potthoff MJ, Inagaki T, Satapati S, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA 2009;106:10853-8. [Crossref] [PubMed]
- Lin Z, Pan X, Wu F, et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation 2015;131:1861-71. [Crossref] [PubMed]
- Lin Z, Tian H, Lam KS, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metabolism 2013;17:779-89. [Crossref] [PubMed]
- Hoofnagle JH, Carithers RL Jr, Shapiro C, et al. Fulminant hepatic failure: summary of a workshop. Hepatology 1995;21:240-52. [PubMed]
- Kullak-Ublick GA, Andrade RJ, Merz M, et al. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut 2017;66:1154-64. [Crossref] [PubMed]
- Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013;369:2525-34. [Crossref] [PubMed]
- Bunchorntavakul C, Reddy KR. Acute Liver Failure. Clinics in Liver Disease. 2017;21:769-92. [Crossref] [PubMed]
- Bower WA, Johns M, Margolis HS, et al. Population-based surveillance for acute liver failure. Am J Gastroenterol 2007;102:2459-63. [Crossref] [PubMed]
- Cordoba J, Dhawan A, Larsen FS, et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol 2017;66:1047-81. [Crossref] [PubMed]
- Yoon E, Babar A, Choudhary M, et al. Acetaminophen-Induced Hepatotoxicity: a Comprehensive Update. J Clin Transl Hepatol 2016;4:131-42. [PubMed]
- Arundel C, Lewis JH. Drug-induced liver disease in 2006. Curr Opin Gastroenterol 2007;23:244-54. [Crossref] [PubMed]
- Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005;42:1364-72. [Crossref] [PubMed]
- Blieden M, Paramore LC, Shah D, et al. A perspective on the epidemiology of acetaminophen exposure and toxicity in the United States. Expert Rev Clin Pharmacol 2014;7:341-8. [Crossref] [PubMed]
- Sheen CL, Dillon JF, Bateman DN, et al. Paracetamol toxicity: epidemiology, prevention and costs to the health-care system. QJM 2002;95:609-19. [Crossref] [PubMed]
- Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 2010;196:369-405. [Crossref] [PubMed]
- Underhill TJ, Greene MK, Dove AF. A comparison of the efficacy of gastric lavage, ipecacuanha and activated charcoal in the emergency management of paracetamol overdose. Arch Emerg Med 1990;7:148-54. [Crossref] [PubMed]
- Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med 2008;359:285-92. [Crossref] [PubMed]
- Bailey B, Amre DK, Gaudreault P. Fulminant hepatic failure secondary to acetaminophen poisoning: a systematic review and meta-analysis of prognostic criteria determining the need for liver transplantation. Crit Care Med 2003;31:299-305. [Crossref] [PubMed]
- Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol 2013;59:1094-106. [Crossref] [PubMed]
- Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg 2001;181:160-6. [Crossref] [PubMed]
- Chen Y, Xie X. Tacrolimus attenuates myocardium damage to the total hepatic ischemia-reperfusion via regulation of the mitochondrial function. J Surg Res 2012;172:e47-54. [Crossref] [PubMed]
- Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl 2003;9:651-63. [Crossref] [PubMed]
- Saidi RF, Hejazii Kenari SK. Challenges of organ shortage for transplantation: solutions and opportunities. Int J Organ Transplant Med 2014;5:87-96. [PubMed]
- Abu-Amara M, Gurusamy KS, Glantzounis G, et al. Pharmacological interventions for ischaemia reperfusion injury in liver resection surgery performed under vascular control. Cochrane Database Syst Rev 2009;248. [PubMed]
- Pantazi E, Bejaoui M, Folch-Puy E, et al. Advances in treatment strategies for ischemia reperfusion injury. Expert Opin Pharmacother 2016;17:169-79. [Crossref] [PubMed]
- Hernaez R, Solà E, Moreau R, et al. Acute-on-chronic liver failure: an update. Gut 2017;66:541-53. [Crossref] [PubMed]
- Gustot T, Fernandez J, Garcia E, et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015;62:243-52. [Crossref] [PubMed]
- Bernal W, Jalan R, Quaglia A, et al. Acute-on-chronic liver failure. Lancet 2015;386:1576-87. [Crossref] [PubMed]
- Stickel F, Datz C, Hampe J, et al. Pathophysiology and Management of Alcoholic Liver Disease: Update 2016. Gut Liver 2017;11:173-88. [Crossref] [PubMed]
- Jalan R, Ginés P, Olson JC, et al. Acute-on chronic liver failure. J Hepatol 2012;57:1336-48. [Crossref] [PubMed]
- Struecker B, Raschzok N, Sauer IM. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol 2014;11:166-76. [Crossref] [PubMed]
- Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426-37,1437.e1-9.
- Karvellas CJ, Lescot T, Goldberg P, et al. Liver transplantation in the critically ill: a multicenter Canadian retrospective cohort study. Crit Care 2013;17:R28. [Crossref] [PubMed]
- Modica S, Petruzzelli M, Bellafante E, et al. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology 2012;142:355-65.e1-4.
- Lin BC, Desnoyers LR. FGF19 and cancer. Adv Exp Med Biol 2012;728:183-94. [Crossref] [PubMed]
- Hartmann P, Hochrath K, Horvath A, et al. Modulation of the intestinal bile acid-FXR-FGF15 axis improves alcoholic liver disease in mice. Hepatology 2018;67:2150-66. [Crossref] [PubMed]
- Zhou M, Learned RM, Rossi SJ, et al. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol Commun 2017;1:1024-42. [Crossref] [PubMed]
- McCormack L, Petrowsky H, Jochum W, et al. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg 2007;245:923-30. [Crossref] [PubMed]
- Kele PG, van der Jagt EJ, Gouw AS, et al. The impact of hepatic steatosis on liver regeneration after partial hepatectomy. Liver Int 2013;33:469-75. [Crossref] [PubMed]
- Hoppe S. Nonalcoholic steatohepatits and liver steatosis modify partial hepatectomy recovery. J Invest Surg 2015;28:24-31. [Crossref] [PubMed]
- Cho JY, Suh KS, Lee HW, et al. Hepatic steatosis is associated with intrahepatic cholestasis and transient hyperbilirubinemia during regeneration after living donor liver transplantation. Transpl Int 2006;19:807-13. [Crossref] [PubMed]
- Fioravanti J, González I, Medina-Echeverz J, et al. Anchoring interferon alpha to apolipoprotein A-I reduces hematological toxicity while enhancing immunostimulatory properties. Hepatology 2011;53:1864-73. [Crossref] [PubMed]
- Murata H, Yagi T, Iwagaki H, et al. Mechanism of impaired regeneration of fatty liver in mouse partial hepatectomy model. J Gastroenterol Hepatol 2007;22:2173-80. [Crossref] [PubMed]
- Hamano M, Ezaki H, Kiso S, et al. Lipid overloading during liver regeneration causes delayed hepatocyte DNA replication by increasing ER stress in mice with simple hepatic steatosis. J Gastroenterol 2014;49:305-16. [Crossref] [PubMed]
- Inaba Y, Furutani T, Kimura K, et al. Growth arrest and DNA damage-inducible 34 regulates liver regeneration in hepatic steatosis in mice. Hepatology 2015;61:1343-56. [Crossref] [PubMed]
- Alvarez-Sola G, Uriarte I, Latasa MU, et al. Engineered fibroblast growth factor 19 protects from acetaminophen-induced liver injury and stimulates aged liver regeneration in mice. Cell Death Dis 2017;8. [Crossref] [PubMed]
- Schmucker DL, Sanchez H. Liver regeneration and aging: a current perspective. Curr Gerontol Geriatr Res 2011;2011. [Crossref] [PubMed]
- Hanawa N, Shinohara M, Saberi B, et al. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem 2008;283:13565-77. [Crossref] [PubMed]
- Win S, Than TA, Zhang J, et al. New insights into the role and mechanism of c-Jun-N-terminal kinase signaling in the pathobiology of liver diseases. Hepatology 2018;67:2013-24. [Crossref] [PubMed]
- Vispute SG, Bu P, Le Y, et al. Activation of GR but not PXR by dexamethasone attenuated acetaminophen hepatotoxicities via Fgf21 induction. Toxicology 2017;378:95-106. [Crossref] [PubMed]
- Ma Q, He X. Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2. Pharmacol Rev 2012;64:1055-81. [Crossref] [PubMed]
- Yu Y, Bai F, Liu Y, et al. Fibroblast growth factor (FGF21) protects mouse liver against D-galactose-induced oxidative stress and apoptosis via activating Nrf2 and PI3K/Akt pathways. Mol Cell Biochem 2015;403:287-99. [Crossref] [PubMed]
- Chen X, Ward SC, Cederbaum AI, et al. Alcoholic fatty liver is enhanced in CYP2A5 knockout mice: The role of the PPARα-FGF21 axis. Toxicology 2017;379:12-21. [Crossref] [PubMed]
- Lee JH, Kang YE, Chang JY, et al. An engineered FGF21 variant, LY2405319, can prevent non-alcoholic steatohepatitis by enhancing hepatic mitochondrial function. Am J Transl Res 2016;8:4750-63. [PubMed]
- Gaich G, Chien JY, Fu H, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metabolism 2013;18:333-40. [Crossref] [PubMed]


