The basics of respiratory mechanics: ventilator-derived parameters
Introduction
Mechanical ventilation is a life-support system used to maintain adequate lung function in patients who are critically ill or undergoing general anesthesia (1,2); however, it may cause lung damage. The benefits and harms of mechanical ventilation depend not only on the adjustment of ventilator parameters, but also on the interpretation of ventilator-derived parameters, which should be used to guide ventilatory strategies. The basis of this process relies on the interaction between physical forces acting on lung structures during mechanical ventilation adjusted by the operator and the lung and chest wall mechanics of the patient (3). Once the inputs—tidal volume (VT), positive end-expiratory pressure (PEEP), respiratory rate (RR), and inspiratory airflow (V’)—have been adjusted, the information obtained from the mechanical ventilator (the outputs or ventilator-derived parameters) can be examined. Regardless of ventilator mode, the following ventilator-derived parameters should be measured in order to mitigate harmful effects (2,4): intrinsic PEEP (PEEPi), peak (Ppeak) and plateau (Pplat) pressures, driving pressure (ΔP), and transpulmonary pressure (PL). During assisted mechanical ventilation, in addition to these parameters, the pressure generated 100 ms after onset of inspiratory effort (P0.1) and pressure-time product per minute (PTP/min) should also be evaluated. In this review, we will discuss the ventilator parameters adjusted by the operator (inputs) and ventilator parameters obtained after interaction with respiratory system structures during mechanical ventilation (outputs). Moreover, new ventilator-derived parameters, such as mechanical energy, mechanical power, and intensity, will be discussed in light of recent evidence (5-7).
Inputs: ventilator parameters set by the operator
Tidal volume (VT)
In both uninjured and injured lungs, the use of low VT has been preferred over high VT.
In patients under general anesthesia, no association has been observed between VT and postoperative pulmonary complications (PPCs) (8). Additionally, pressure-controlled ventilation (PCV) has been compared to volume-controlled ventilation (VCV), focusing on PPCs; this comparison is important to distinguish the potential role of strict control of VT during VCV. The frequency of PPCs was higher in PCV than in VCV. This could be attributed to the difficulty in controlling VT during PCV, thus highlighting the importance of VT.
In the emergency department, mechanically ventilated patients with injured and uninjured lungs could also benefit from the use of low VTs (9).
In the intensive care unit (ICU), even though two meta-analyses suggest that patients with uninjured lungs could benefit from ventilation with low VT (10,11), a prospective study reported no association between VT and outcomes (12), which may be attributed to the fact that VT in this study was much lower than in the aforementioned meta-analyses (10,11). In out-of-hospital cardiac arrest, VT reduction has been associated with favorable neurocognitive outcome and more ventilator-free days (13). In short, the benefit of reduced VT in ICU patients with uninjured lungs remains unclear. Two ongoing randomized clinical trials, Protective Ventilation in patients without ARDS at start of ventilation (PReVENT) (14) and Preventive Strategies in Acute Respiratory Distress Syndrome (EPALI) (Clinicaltrials.org registration number: NCT02070666), may elucidate this issue.
In patients with the acute respiratory distress syndrome (ARDS), predicted body weight (PBW), taking into account both sex and height, has been used to set VT (15-17). The following PBW equations have been used: men, 50.0+0.905× (height in cm) −152.4; women: 45.5+0.905× (height in cm) −152.4. To mitigate the risk of ventilator induced lung injury (VILI) in ARDS patients, the National Institute of Health ARDS Network protocol suggests the use of VT =6 mL/kg PBW and Pplat limited to 30 cmH2O. If Pplat exceeds 30 cmH2O with a VT of 6 mL/kg PBW, the protocol recommends a reduction in VT (to 4–5 mL/kg PBW) if pHa >7.15. Since ARDS lungs present great variability due to edema, atelectasis, and consolidation, VT should probably be set according to aerated lung volume, e.g., functional residual capacity (FRC) or total lung capacity (TLC) (18-20).
Nevertheless, further studies are required to evaluate the safe limit of FRC and TLC when used to set VT. In this line, in patients with severe ARDS and very low lung compliance, even setting VT below 6 mL/kg PBW can result in high strain (VT/FRC) (19). This scenario may be considered unsafe; thus, rescue therapies are needed, such as extracorporeal support (21).
Additionally, VT should be set according to ΔP [Pplat-PEEP or VT/Crs (respiratory system compliance)]. Since Crs is directly related to lung size, ΔP reflects the level of VT in relation to the aerated lung volume. However, in the presence of reduced chest wall mechanics, ΔP does not reflect VT. In this line, considering the same ΔP, a patient with a stiff chest wall has less lung overinflation than one with a normal chest wall (22). Therefore, transpulmonary driving pressure (ΔPL, the difference in transpulmonary pressure between end-expiration and end-inspiration) (23) should be evaluated, and VT could be limited to keep ΔPL in a safe range (19,24).
Positive end-expiratory pressure (PEEP)
PEEP is the alveolar pressure above the atmospheric pressure at end-expiration. PEEP applied through mechanical ventilation (i.e., extrinsic PEEP) allows delivery of positive pressure at the end of expiration to prevent unstable lung units from collapsing. Low levels of PEEP (3 to 5 cmH2O) are routinely used in patients on mechanical ventilation. This practice is important to: (I) keep lungs open at the end of expiration, thus promoting alveolar stabilization (25); (II) prevent opening and closing of distal small airways and alveolar units (26); and (III) increase lymphatic flow through the thoracic duct, which may facilitate drainage of lung edema (27). However, higher levels of PEEP may cause regional overdistension and impairment of cardiac performance (28). The pros and cons of PEEP depend on the degree of lung injury (29).
In patients under general anesthesia, intraoperative mechanical ventilation with VT =8 mL/kg and high PEEP (12 cmH2O), when compared with low PEEP (2 cmH2O), does not prevent PPCs, as shown in the Protective Ventilation using High versus Low positive end-expiratory pressure (PROVHILO) trial (30). Further research is required to evaluate moderate levels of PEEP (5–8 cmH2O).
In the emergency department, the use of higher PEEP levels was associated with improvement in ventilator- and hospital-free days in patients with ARDS (9) and uninjured lungs (31).
In ICU patients with uninjured lungs, a meta-analysis reported that benefit from PEEP is lacking in terms of duration of mechanical ventilation and mortality rate (32). In ICU patients at risk of ARDS, higher PEEP levels are required than in those without ARDS risk (12). More recently, ICU patients after cardiac surgery were found to exhibit fewer lung complications with high PEEP (33). Certainly, further studies are required to compare low vs. high PEEP levels in ICU patients without ARDS.
Three major studies have assessed high vs. low PEEP levels combined with low VT for ARDS patients (16,17,34). In the ALVEOLI trial (34), mortality did not differ between low and high PEEP levels. High PEEP resulted in improved oxygenation (17) as well as more ventilator-free days and organ failure-free days (16); however, mortality rate did not differ between PEEP arms. A meta-analysis that used the data from the aforementioned three trials found that higher PEEP levels were associated with improved survival in severe ARDS (35). In moderate ARDS, lower PEEP (<12 cmH2O), compared to higher PEEP, was associated with greater risk of hospital mortality (26%) (36). In a recent randomized clinical trial comparing individualized PEEP titration after recruitment maneuvers (RMs) vs. low PEEP without RMs in patients with moderate-to-severe ARDS, an increase in 28-day mortality was observed in the recruited group (37).
Several strategies have been used to determine optimal PEEP, such as: (I) evaluation of the lower inflection point of the pressure-volume curve, which reflects the transition from low to high compliance, and application of PEEP 2 cmH2O greater than this point; (II) the use of algorithms combining PEEP and fraction of inspired oxygen (FiO2); and (III) measurement of transpulmonary pressure with an esophageal catheter (38). Certainly, the best approach is to individualize PEEP for each patient.
Respiratory rate
Respiratory rate must be adjusted during mechanical ventilation to maintain a minute volume appropriate to the patient’s metabolic demands. Although higher RR is often needed to maintain CO2 levels within safe range (39), it can alter the inspiratory-to-expiratory ratio, thus leading to intrinsic PEEP due to short expiratory time. In this context, Vieillard-Baron et al. compared two levels of RR—15 breaths per minute (bpm) vs. 30 bpm—while maintaining lower Pplat (<25 cmH2O). No difference in PaCO2 due to increased intrinsic PEEP or dead space ventilation was observed between groups (40). Increased RR may also cause lung damage due to cyclic recruitment/derecruitment.
Inspiratory airflow
Inspiratory airflow must be adjusted during mechanical ventilation, since it may also cause lung damage (41-43). The mechanism whereby inspiratory airflow contributes to lung injury seems to be influenced by the viscoelastic properties of lung tissue. High inspiratory airflow enhances damage to the lung parenchyma because the viscoelastic accommodation has no time to dissipate damaging forces when inflation occurs rapidly. This type of mechanism of injury usually occurs in asymmetrical lungs.
High inspiratory airflow is an important determinant of pulmonary stress, since it enhances the transmission of kinetic energy to lung structures, increases shear stress parallel to the surface of the airways and alveolar walls, leads to deformation of the pulmonary parenchyma and bronchial epithelial cells, and releases pro-fibrogenic (43) and pro-inflammatory (44) mediators. Therefore, controlling inspiratory airflow might provide additional lung protection (43,44).
Outputs: ventilator parameters obtained as a result of the interaction between mechanical ventilator and respiratory system
During mechanical ventilation, several ventilator-derived parameters should be monitored: PEEPi, Ppeak, Pplat, ΔP, PL, P0.1, PTP/min, mechanical energy, mechanical power, and intensity.
Intrinsic PEEP
Intrinsic PEEP (PEEPi) reflects the residual pressure when the expiratory phase may not be completed to full exhalation. This residual pressure is higher than the point of equilibrium of the respiratory system’s elastic properties (45). One easy form to detect its presence is to perform an expiratory pause and check the end-expiratory pressure. PEEPi is usually associated with obstructive diseases (46), but may be present during other conditions; therefore, it is considered an important ventilator-derived parameter for monitoring. For example, obese patients under mechanical ventilation are prone to developing PEEPi, mainly in the supine position. Both external PEEP application and changing position (beach-chair) may alter PEEPi (47).
Peak pressure
Peak pressure is the maximum pressure measured at end inspiration. Ppeak includes the elastic and resistive components (airway, lung tissue, and equipment, e.g., endotracheal tube). At bedside, the difference between Ppeak and Pplat can be easily visualized during an inspiratory pause in controlled mechanical ventilation with constant airflow. Immediately after the inspiratory pause, a rapid airway pressure decay, which represents the pressure dissipated to overcome airway resistance, is observed. The difference between Ppeak and Pplat divided by the airflow is the airway resistance. In normal subjects, airway resistance values do not exceed 15–20 cmH2O/L/s under controlled mechanical ventilation (48). Several factors can modify Ppeak, such as endotracheal tube diameter (49,50), airflow intensity, plugging, or bronchospasm.
During controlled mechanical ventilation, Ppeak depends on VT, RR, and airflow, whereas during assisted mechanical ventilation, the patient’s effort also contributes to Ppeak.
In a multicenter, prospective cohort study of 2,377 patients with severe respiratory failure, conducted in 459 ICUs from 50 countries across five continents (36), the authors reported the importance of monitoring Ppeak besides other ventilator-derived parameters. Higher Ppeak, especially above 40 cmH2O, is associated with increased mortality rates (51).
Plateau pressure
Plateau pressure can be measured during an inspiratory pause when the respiratory muscles are relaxed and is equal to alveolar pressure when airflow is zero. Pplat can be affected by changes in VT and Crs, but not by changes in airflow and airway resistance (52).
In ICU patients without ARDS, lower Pplat values associated with VT ≤7 mL/kg PBW lead to reduced PPCs and a trend toward increased survival (P=0.052) (11). In ARDS patients, Pplat <30 cmH2O was associated with lower mortality (15). An observational study with ARDS patients suggested that Pplat <28 cmH2O is more beneficial in those with a large percentage of non-aerated lung tissue (53). More recently, in patients with severe ARDS, the LUNG SAFE study (36) reported that Pplat <25 cmH2O was not associated with decreased risk of hospital mortality. However, patients with a median Pplat ≥23 cmH2O on day 1 of ARDS diagnosis had higher mortality.
Driving pressure
Driving pressure is defined as Pplat-PEEP or VT normalized to Crs (23,54,55). During intraoperative ventilation, ΔP seems to be an important parameter for the optimization of mechanical ventilation (8,12).
In mechanically ventilated ICU patients without ARDS (55), ΔP was not associated with hospital mortality. The authors attributed this result to the fact that Crs was not a major risk factor for mortality in those patients without ARDS. Conversely, Tejerina et al. (56) showed that, in patients with brain injury but uninjured lungs, low ÄP resulted in a better outcome.
In a study of ARDS patients, ΔP was considered the variable most strongly associated with survival, as opposed to VT and PEEP (54). The authors observed that increasing PEEP level for a short period could lead to different changes in ΔP. If the increase in PEEP level leads to increased aeration of lung tissue through recruitment, a decrease in ΔP is expected. On the other hand, if PEEP increases and does not recruit lung tissue, the lungs may become overstretched, and ΔP may remain unchanged or even increase over time (Figure 1).
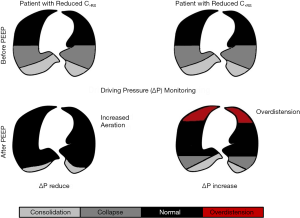
The LUNG SAFE study (36) showed that ΔP <14 cmH2O was associated with decreased risk of hospital mortality in patients with moderate-to-severe ARDS.
Transpulmonary pressure
Transpulmonary pressure, by definition, is the difference between airway pressure (Paw) and pleural pressure (Ppl). In the clinical setting, esophageal manometry is the only clinically available method to separate airway pressure applied to the respiratory system into its chest wall (i.e., Ppl) and lung component (PL) (57-59). PL measurement has been proposed because it can determine the pressure required to keep the lungs open (38,60,61) and it can assess inspiratory effort (62,63). In influenza A(H1N1)-associated ARDS, Grasso et al. (61) observed that the pressure applied to the airways was not transmitted to the lung parenchyma but dissipated against a stiff chest wall, providing further evidence of the importance of measuring PL.
During assisted mechanical ventilation, the esophageal catheter may not cover the entire vertical gradient while respiratory muscle activity is present. In this context, Yoshida et al. (64) showed that esophageal pressure variation significantly underestimated pleural pressure variation in dependent regions. In addition, directly measured swings in pleural pressure (−14.9) were significantly greater in dependent lung than swings in Pes (−7.1). Esophageal pressure may underestimate the local pleural pressure, especially in those areas near the diaphragm which present higher degrees of PL.
Transpulmonary driving pressure (ΔPL)
The transpulmonary driving pressure (ΔPL) is defined as the difference between PL at end-inspiration (PLend-insp) and PL at end-expiration (PLend-exp). It reflects the distending pressure taken by the lungs when VT is delivered. The use of ΔPL offers some advantages. First, ΔPL removes the stress caused by PEEP, which does not necessarily contribute to lung injury and sometimes can mitigate it (65). Second, ΔPL removes the distending pressure taken by the chest wall (66). Hence, it seems that ΔPL might be a better surrogate of lung stress and may even be a better predictor of clinical outcomes than ΔP (67). ΔPL is calculated as:
ΔPL = (PPLAT – PESO, end-insp) – (PEEPTOT − PESO, end-exp) [1]
In experimental ARDS, low PL did not increase lung inflammation, despite leading to alveolar collapse. Intermediate levels of ΔPL reduced alveolar collapse, increased overdistension, and resulted in alveolar instability. At high PL levels, alveolar hyperinflation was detected, but no further lung inflammation was observed (23). This study highlighted the importance of permissive atelectasis to protect lung damage, as recently published (68) and discussed in two editorials (69,70).
PL is also an important ventilator parameter to be monitored during assisted mechanical ventilation. Bellani et al. tested the hypothesis that, for a given inspired volume and flow, and for the same mechanical properties (i.e., compliance and resistance) of the lung, ΔPL during assisted and controlled mechanical ventilation should not differ within the same patient (71). They found no difference in ΔPL at comparable volumes and flows. However, the authors pointed out that, if assisted breaths contributed to lung damage, this would not be due solely to ΔPL; the vertical gradient leading to different local pleural pressures and, ultimately, local ΔPL ranges should also be acknowledged (64,72).
Esophageal pressure generated 100 ms after onset of inspiratory effort (P0.1)
The esophageal pressure generated 100 ms after the onset of an occluded inspiratory effort (P0.1) has been used as a measurement of respiratory drive (73), and it could be used to optimize the level of pressure support in individual patients (74). In a recent prospective, randomized, crossover physiologic study, P0.1 was evaluated in the presence of different degrees of inspiratory efforts in patients recovering from acute respiratory failure (75). Inspiratory effort was found to correlate strongly with P0.1. Therefore, this parameter may have yet-unrecognized importance as a marker of respiratory drive during mechanical ventilation, and efforts should be made to increase awareness of its potential utility (76).
Pressure-time product per minute
Pressure-time product is a measure of the mechanical work of breathing. By integrating the pressure developed by the respiratory muscles over the duration of the contraction (i.e., chest wall elastic recoil pressure), it is possible to obtain the respiratory PTP. Field et al. (77) found that the oxygen consumption of the respiratory muscles is only weakly correlated with the mechanical work of breathing (the product ΔP·ΔV), whereas it is well reflected by the PTP. PTP takes into account the isometric phase of muscle contraction, thus representing a good indicator of energy expenditure (78). A common way of expressing PTP is through standardization by the sample period of a respiratory cycle (TTOT).
New ventilator-derived parameters: markers of patient–machine interaction
Mechanical energy
The energy delivered per breath to the airways and lungs is defined as the area between the inspiratory limb of pressure (x) vs. the volume axis (y), measured in joules (J) (79) (Figure 2).
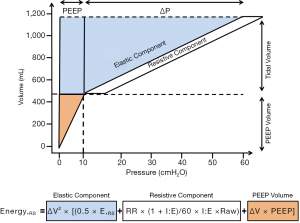
Two equations have been proposed to calculate mechanical energy: one simple (80) and another more complex (7). If properly adjusted, both should yield similar results. Nevertheless, some technical differences between the two should be addressed.
Simple equation:
EnergyL = ΔPL2/EL [2]
where ΔPL is the transpulmonary driving pressure and EL is the lung elastance.
Complex equation:
EnergyL = ΔV2 × [(0.5× ERS + RR × (1+ I:E)/60× I:E × Raw) + ΔV × PEEP] [3]
where ΔV is the variation of tidal volume, ERS is the respiratory system elastance, I:E is the inspiratory to expiratory ratio, and Raw is the airway resistance.
The simplified equation can be easily used in the clinical setting (5,80,81). This equation computes the most important component (driving mechanical power), without taking into account resistive properties or the contribution PEEP, unlike the equation proposed by Gattinoni et al. (7). However, it is difficult to directly link the mechanical energy dissipated in the proximal airways to alveolar injury. The addition of PEEP to the complex equation takes into account the contribution of static strain, which is associated with potential energy storage within the elastic tissues of the respiratory system (81).
Mechanical power and intensity
Mechanical power represents the mechanical energy multiplied by the RR. In a previous study (6), different mechanical power values were applied to the respiratory system in healthy pigs by changing the RR while keeping the VT and PL constant, aiming to identify a mechanical power threshold for lung damage. The authors reported development of edema only when the delivered transpulmonary mechanical power exceeded 12.1 J/min. In the presence of lung damage, the ventilated lung area is reduced, thus requiring greater driving pressure and airflow. This, in turn, increases the mechanical power delivered without changes in VT.
The so-called intensity (i.e., mechanical power normalized to the lung tissue) should also be considered. Depending on the mechanical power, intensity may be comparable in volutrauma and atelectrauma (5). If power increases without changes in lung surface area, the intensity will be higher; on the other hand, if both power and lung surface area increase (e.g., due to lung recruitment), the intensity may reduce or remain constant.
Conclusions
The benefits and harms of mechanical ventilation in critically ill patients with uninjured or injured lungs, as well as in patients undergoing general anesthesia, depend not only on ventilator settings, but also on the interpretation of ventilator-derived parameters. Both parameters adjusted by the operator (VT, PEEP, RR, and V’) and ventilator-derived parameters (PEEPi, Ppeak, Pplat, ΔP, PL, mechanical energy, mechanical power, intensity, P0.1, and PTP) need to be strictly monitored at bedside, in order to develop a case-by-case approach to mechanical ventilation. Furthermore, additional clinical studies are required to ascertain the safe thresholds of each of these parameter in injured and uninjured lungs.
Acknowledgements
The authors would like to express their gratitude to Mrs. Moira Elizabeth Schottler and Mr. Filippe Vasconcellos for their assistance in editing the manuscript.
Funding: This work was supported by grants from the Carlos Chagas Filho Rio de Janeiro State Research Foundation (FAPERJ) (grant number E-26/103.118/2), Rio de Janeiro, Brazil; and the Brazilian Council for Scientific and Technological Development (CNPq) (grant number 471438/2012-0), Brasília, Brazil.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126-36. [Crossref] [PubMed]
- Silva PL, Pelosi P, Rocco PR. Optimal mechanical ventilation strategies to minimize ventilator-induced lung injury in non-injured and injured lungs. Expert Rev Respir Med 2016;10:1243-5. [Crossref] [PubMed]
- Plataki M, Hubmayr RD. The physical basis of ventilator-induced lung injury. Expert Rev Respir Med 2010;4:373-85. [Crossref] [PubMed]
- Silva PL, Negrini D, Rocco PR. Mechanisms of ventilator-induced lung injury in healthy lungs. Best Pract Res Clin Anaesthesiol 2015;29:301-13. [Crossref] [PubMed]
- Samary CS, Silva PL, Gama de Abreu M, et al. Ventilator-induced Lung Injury: Power to the Mechanical Power. Anesthesiology 2016;125:1070-1. [Crossref] [PubMed]
- Cressoni M, Gotti M, Chiurazzi C, et al. Mechanical Power and Development of Ventilator-induced Lung Injury. Anesthesiology 2016;124:1100-8. [Crossref] [PubMed]
- Gattinoni L, Tonetti T, Cressoni M, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med 2016;42:1567-75. [Crossref] [PubMed]
- Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS - an observational study in 29 countries. Eur J Anaesthesiol 2017;34:492-507. [Crossref] [PubMed]
- Fuller BM, Ferguson IT, Mohr NM, et al. A Quasi-Experimental, Before-After Trial Examining the Impact of an Emergency Department Mechanical Ventilator Protocol on Clinical Outcomes and Lung-Protective Ventilation in Acute Respiratory Distress Syndrome. Crit Care Med 2017;45:645-52. [Crossref] [PubMed]
- Serpa Neto A, Simonis FD, Barbas CS, et al. Association between tidal volume size, duration of ventilation, and sedation needs in patients without acute respiratory distress syndrome: an individual patient data meta-analysis. Intensive Care Med 2014;40:950-7. [Crossref] [PubMed]
- Neto AS, Simonis FD, Barbas CS, et al. Lung-Protective Ventilation With Low Tidal Volumes and the Occurrence of Pulmonary Complications in Patients Without Acute Respiratory Distress Syndrome: A Systematic Review and Individual Patient Data Analysis. Crit Care Med 2015;43:2155-63. [Crossref] [PubMed]
- Neto AS, Barbas CSV, Simonis FD, et al. Epidemiological characteristics, practice of ventilation, and clinical outcome in patients at risk of acute respiratory distress syndrome in intensive care units from 16 countries (PRoVENT): an international, multicentre, prospective study. Lancet Respir Med 2016;4:882-93. [Crossref] [PubMed]
- Beitler JR, Ghafouri TB, Jinadasa SP, et al. Favorable Neurocognitive Outcome with Low Tidal Volume Ventilation after Cardiac Arrest. Am J Respir Crit Care Med 2017;195:1198-206. [Crossref] [PubMed]
- Simonis FD, Binnekade JM, Braber A, et al. PReVENT--protective ventilation in patients without ARDS at start of ventilation: study protocol for a randomized controlled trial. Trials 2015;16:226. [Crossref] [PubMed]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:646-55. [Crossref] [PubMed]
- Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:637-45. [Crossref] [PubMed]
- Gattinoni L, Marini JJ, Pesenti A, et al. The "baby lung" became an adult. Intensive Care Med 2016;42:663-73. [Crossref] [PubMed]
- Chiumello D, Carlesso E, Cadringher P, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med 2008;178:346-55. [Crossref] [PubMed]
- Brower RG, Hubmayr RD, Slutsky AS. Lung stress and strain in acute respiratory distress syndrome: good ideas for clinical management? Am J Respir Crit Care Med 2008;178:323-4. [Crossref] [PubMed]
- Kopp R, Dembinski R, Kuhlen R. Role of extracorporeal lung assist in the treatment of acute respiratory failure. Minerva Anestesiol 2006;72:587-95. [PubMed]
- Sahetya SK, Mancebo J, Brower RG. Fifty Years of Research in ARDS. Vt Selection in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017;196:1519-25. [Crossref] [PubMed]
- Samary CS, Santos RS, Santos CL, et al. Biological Impact of Transpulmonary Driving Pressure in Experimental Acute Respiratory Distress Syndrome. Anesthesiology 2015;123:423-33. [Crossref] [PubMed]
- Protti A, Cressoni M, Santini A, et al. Lung stress and strain during mechanical ventilation: any safe threshold? Am J Respir Crit Care Med 2011;183:1354-62. [Crossref] [PubMed]
- Albert RK. The role of ventilation-induced surfactant dysfunction and atelectasis in causing acute respiratory distress syndrome. Am J Respir Crit Care Med 2012;185:702-8. [Crossref] [PubMed]
- Slutsky AS, Villar J, Pesenti A. Happy 50th birthday ARDS! Intensive Care Med 2016;42:637-9. [Crossref] [PubMed]
- Fernandez Mondejar E, Vazquez Mata G, Cardenas A, et al. Ventilation with positive end-expiratory pressure reduces extravascular lung water and increases lymphatic flow in hydrostatic pulmonary edema. Crit Care Med 1996;24:1562-7. [Crossref] [PubMed]
- Retamal J, Borges JB, Bruhn A, et al. Open lung approach ventilation abolishes the negative effects of respiratory rate in experimental lung injury. Acta Anaesthesiol Scand 2016;60:1131-41. [Crossref] [PubMed]
- Passaro CP, Silva PL, Rzezinski AF, et al. Pulmonary lesion induced by low and high positive end-expiratory pressure levels during protective ventilation in experimental acute lung injury. Crit Care Med 2009;37:1011-7. [Crossref] [PubMed]
- Hemmes SN, Gama de Abreu M, Pelosi P, et al. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014;384:495-503. [Crossref] [PubMed]
- Fuller BM, Ferguson IT, Mohr NM, et al. Lung-Protective Ventilation Initiated in the Emergency Department (LOV-ED): A Quasi-Experimental, Before-After Trial. Ann Emerg Med 2017;70:406-18.e4. [Crossref] [PubMed]
- Serpa Neto A, Filho RR, Cherpanath T, et al. Associations between positive end-expiratory pressure and outcome of patients without ARDS at onset of ventilation: a systematic review and meta-analysis of randomized controlled trials. Ann Intensive Care 2016;6:109. [Crossref] [PubMed]
- Costa Leme A, Hajjar LA, Volpe MS, et al. Effect of Intensive vs Moderate Alveolar Recruitment Strategies Added to Lung-Protective Ventilation on Postoperative Pulmonary Complications: A Randomized Clinical Trial. JAMA 2017;317:1422-32. [Crossref] [PubMed]
- Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-36. [Crossref] [PubMed]
- Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865-73. [Crossref] [PubMed]
- Laffey JG, Bellani G, Pham T, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med 2016;42:1865-76. [Crossref] [PubMed]
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial I, Cavalcanti AB, Suzumura EA, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017;318:1335-45. [Crossref] [PubMed]
- Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008;359:2095-104. [Crossref] [PubMed]
- Wiedemann HP, Arroliga AC. Acute respiratory distress syndrome: low-stretch ventilation improves survival. Cleve Clin J Med 2000;67:435-40. [Crossref] [PubMed]
- Vieillard-Baron A, Prin S, Augarde R, et al. Increasing respiratory rate to improve CO2 clearance during mechanical ventilation is not a panacea in acute respiratory failure. Crit Care Med 2002;30:1407-12. [Crossref] [PubMed]
- Rich PB, Reickert CA, Sawada S, et al. Effect of rate and inspiratory flow on ventilator-induced lung injury. J Trauma 2000;49:903-11. [Crossref] [PubMed]
- Maeda Y, Fujino Y, Uchiyama A, et al. Effects of peak inspiratory flow on development of ventilator-induced lung injury in rabbits. Anesthesiology 2004;101:722-8. [Crossref] [PubMed]
- Garcia CS, Abreu SC, Soares RM, et al. Pulmonary morphofunctional effects of mechanical ventilation with high inspiratory air flow. Crit Care Med 2008;36:232-9. [Crossref] [PubMed]
- Kotani M, Kotani T, Li Z, et al. Reduced inspiratory flow attenuates IL-8 release and MAPK activation of lung overstretch. Eur Respir J 2004;24:238-46. [Crossref] [PubMed]
- Marini JJ. Dynamic hyperinflation and auto-positive end-expiratory pressure: lessons learned over 30 years. Am J Respir Crit Care Med 2011;184:756-62. [Crossref] [PubMed]
- Caramez MP, Borges JB, Tucci MR, et al. Paradoxical responses to positive end-expiratory pressure in patients with airway obstruction during controlled ventilation. Crit Care Med 2005;33:1519-28. [Crossref] [PubMed]
- Lemyze M, Mallat J, Duhamel A, et al. Effects of sitting position and applied positive end-expiratory pressure on respiratory mechanics of critically ill obese patients receiving mechanical ventilation. Crit Care Med 2013;41:2592-9. [Crossref] [PubMed]
- MacIntyre NR, Cook DJ, Ely EW Jr, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Crit Care Med. Chest 2001;120:375S-95S. [Crossref] [PubMed]
- Bock KR, Silver P, Rom M, et al. Reduction in tracheal lumen due to endotracheal intubation and its calculated clinical significance. Chest 2000;118:468-72. [Crossref] [PubMed]
- Rocco PR, Zin WA. Modelling the mechanical effects of tracheal tubes in normal subjects. Eur Respir J 1995;8:121-6. [Crossref] [PubMed]
- Kregenow DA, Rubenfeld GD, Hudson LD, et al. Hypercapnic acidosis and mortality in acute lung injury. Crit Care Med 2006;34:1-7. [Crossref] [PubMed]
- Lucangelo U, Bernabe F, Blanch L. Lung mechanics at the bedside: make it simple. Curr Opin Crit Care 2007;13:64-72. [Crossref] [PubMed]
- Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 2007;175:160-6. [Crossref] [PubMed]
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Schmidt MFS, Amaral A, Fan E, et al. Driving Pressure and Hospital Mortality in Patients Without ARDS: A Cohort Study. Chest 2018;153:46-54. [Crossref] [PubMed]
- Tejerina E, Pelosi P, Muriel A, et al. Association between ventilatory settings and development of acute respiratory distress syndrome in mechanically ventilated patients due to brain injury. J Crit Care 2017;38:341-5. [Crossref] [PubMed]
- Staffieri F, Stripoli T, De Monte V, et al. Physiological effects of an open lung ventilatory strategy titrated on elastance-derived end-inspiratory transpulmonary pressure: study in a pig model*. Crit Care Med 2012;40:2124-31. [Crossref] [PubMed]
- Fumagalli J, Berra L, Zhang C, et al. Transpulmonary Pressure Describes Lung Morphology During Decremental Positive End-Expiratory Pressure Trials in Obesity. Crit Care Med 2017;45:1374-81. [Crossref] [PubMed]
- Yoshida T, Amato MBP, Grieco DL, et al. Esophageal Manometry and Regional Transpulmonary Pressure in Lung Injury. Am J Respir Crit Care Med 2018;197:1018-26. [Crossref] [PubMed]
- Loring SH, Pecchiari M, Della Valle P, et al. Maintaining end-expiratory transpulmonary pressure prevents worsening of ventilator-induced lung injury caused by chest wall constriction in surfactant-depleted rats. Crit Care Med 2010;38:2358-64. [Crossref] [PubMed]
- Grasso S, Terragni P, Birocco A, et al. ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med 2012;38:395-403. [Crossref] [PubMed]
- Yoshida T, Uchiyama A, Matsuura N, et al. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med 2012;40:1578-85. [Crossref] [PubMed]
- Grieco DL, Chen L, Brochard L. Transpulmonary pressure: importance and limits. Ann Transl Med 2017;5:285. [Crossref] [PubMed]
- Yoshida T, Torsani V, Gomes S, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med 2013;188:1420-7. [Crossref] [PubMed]
- Protti A, Andreis DT, Monti M, et al. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med 2013;41:1046-55. [Crossref] [PubMed]
- Cortes-Puentes GA, Keenan JC, Adams AB, et al. Impact of Chest Wall Modifications and Lung Injury on the Correspondence Between Airway and Transpulmonary Driving Pressures. Crit Care Med 2015;43:e287-95. [Crossref] [PubMed]
- Baedorf Kassis E, Loring SH, Talmor D. Mortality and pulmonary mechanics in relation to respiratory system and transpulmonary driving pressures in ARDS. Intensive Care Med 2016;42:1206-13. [Crossref] [PubMed]
- Guldner A, Braune A, Ball L, et al. The authors reply. Crit Care Med 2017;45:e328-9. [Crossref] [PubMed]
- Sahetya SK, Brower RG. Lung Recruitment and Titrated PEEP in Moderate to Severe ARDS: Is the Door Closing on the Open Lung? JAMA 2017;318:1327-9. [Crossref] [PubMed]
- Villar J, Suarez-Sipmann F, Kacmarek RM. Should the ART trial change our practice? J Thorac Dis 2017;9:4871-7. [Crossref] [PubMed]
- Bellani G, Grasselli G, Teggia-Droghi M, et al. Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study. Crit Care 2016;20:142. [Crossref] [PubMed]
- Yoshida T, Brochard L. Ten tips to facilitate understanding and clinical use of esophageal pressure manometry. Intensive Care Med 2018;44:220-2. [Crossref] [PubMed]
- Elliott MW, Mulvey DA, Green M, et al. An evaluation of P0.1 measured in mouth and oesophagus, during carbon dioxide rebreathing in COPD. Eur Respir J 1993;6:1055-9. [PubMed]
- Alberti A, Gallo F, Fongaro A, et al. P0.1 is a useful parameter in setting the level of pressure support ventilation. Intensive Care Med 1995;21:547-53. [Crossref] [PubMed]
- Rittayamai N, Beloncle F, Goligher EC, et al. Effect of inspiratory synchronization during pressure-controlled ventilation on lung distension and inspiratory effort. Ann Intensive Care 2017;7:100. [Crossref] [PubMed]
- Telias I, Damiani F, Brochard L. The airway occlusion pressure (P0.1) to monitor respiratory drive during mechanical ventilation: increasing awareness of a not-so-new problem. Intensive Care Med 2018;44:1532-5. [Crossref] [PubMed]
- Field S, Sanci S, Grassino A. Respiratory muscle oxygen consumption estimated by the diaphragm pressure-time index. J Appl Physiol Respir Environ Exerc Physiol 1984;57:44-51. [PubMed]
- Sassoon CS, Mahutte CK. Work of breathing during mechanical ventilation. In: Marini JJ SA, editor. Physiological Basis of Ventilatory Support. New York: Marcel Dekker; 1998, p. 261-310.
- Tonetti T, Vasques F, Rapetti F, et al. Driving pressure and mechanical power: new targets for VILI prevention. Ann Transl Med 2017;5:286. [Crossref] [PubMed]
- Guerin C, Papazian L, Reignier J, et al. Effect of driving pressure on mortality in ARDS patients during lung protective mechanical ventilation in two randomized controlled trials. Critical Care 2016;20:384. [Crossref] [PubMed]
- Marini JJ, Jaber S. Dynamic predictors of VILI risk: beyond the driving pressure. Intensive Care Med 2016;42:1597-600. [Crossref] [PubMed]


