
Determining association of rho kinase 1 gene polymorphisms with risk of Alzheimer’s disease: a multicenter pilot study
Introduction
Alzheimer’s disease (AD), the most common dementia, is a genetically complex disease which can be caused by genetic and environmental factors, individually and in interaction with each other. Mutations in three genes (APP, PSEN1, and PSEN2) have been identified to be associated with autosomal dominant forms of familial AD (1). As mutations that cause familial AD, other heritable genetic risk factors also contribute to individual’s susceptibilities to AD. For example, Apolipoprotein E (APOE) ε4 allelic variants have been identified (2), and found to be linked to a major increase in the risk for susceptibility to AD in different populations (3). Furthermore, genome-wide association studies (GWAS) have identified more than 20 genetic loci associated with the risk of AD, including BIN1, PICALM, CLU, CR1, MS4A6A and others (4).
Rho kinases (ROCKs) are serine/threonine kinases first identified as downstream effector of Rho GTPase. ROCK genes consist of two paralogs: ROCK1 and ROCK2, sharing 65% homology in amino acid sequences and 92% similarity in kinase domains (5). ROCKs constitute an important intracellular signaling system that participates in the regulation of a multitude of cellular functions, such as proliferation, differentiation, metabolism and apoptosis (6,7). Notably, several studies have suggested that ROCK kinases can induce the processing of APP into the toxic β-amyloid (Aβ) 1-42 peptide and furthermore, inhibitors of ROCKs, such as statins and NSAIDs, can inhibit this toxic APP processing (8,9). ROCK1 is located on chromosome 18 (18q11.1) and is expressed mainly in hypothalamus and hippocampus. Recent research has found that ROCK1 is elevated in mild cognitive impairment (MCI) and AD brain, and knockout of the ROCK1 gene reduced the production of Aβ (10). Similarly, our recent study suggested that ROCK1 increases Aβ clearance by modulating autophagosome formation and is also involved in tau hyperphosphorylation and cytoskeleton disruption by miRNA-146a (11-13). These discoveries elucidated that ROCK1 is involved in modulating metabolism of both Aβ and tau, the constituents of hallmark AD pathologies.
Interestingly, there are several published studies in regard to the relationship between ROCK1 gene polymorphisms and diseases that threaten the health of people (14-18) With increasing evidence for a molecular mechanism of ROCK1 in AD pathogenesis, ROCK1 genetic variance as a potential contributor to AD risk is still not completely understood. Here, we put forward a hypothesis that ROCK1 gene polymorphisms affect potential risk for AD, and we test this with a case-control study (n=501) to determine the prevalence of the common single-nucleotide polymorphisms (SNPs) of ROCK1 (rs35996865, rs11873284, and rs2127958) among healthy and AD patients in the Chinese Han population, and investigate the association between these polymorphisms and AD risk.
Methods
Study population
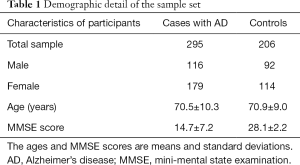
A total of 295 AD patients of Chinese Han ethnicity (179 women and 116 men; 70.5±10.3 years old were recruited; the Mini-Mental State Examination (MMSE) score mean for these patients was 14.7±7.2) were enrolled from the outpatient Clinic at the Department of Neurology, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, and at the Department of Geriatric Psychiatry, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, and Department of Neurology & Institute of Neurology, Huashan Hospital, Fudan University. All subjects received a detailed neurological examination and underwent a psychiatric interview. The patients had a clinical diagnosis of probable dementia of Alzheimer type according to NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association). Two hundred and six gender, age, and ethnic background-matched non-demented elderly controls (NEC) (114 women and 92 men; 70.9±9.0 years old at the recruitment; average MMSE score, 28.1±2.2) were also recruited. The makeup of male and female subjects did not vary significantly (P>0.05) (Table 1). The NEC subjects were free of neurological or psychiatric disorders by medical history, physical examinations, laboratory examinations, and with a MMSE score over 26 (Table 1). All AD patients and NEC were unrelated Chinese Han. Informed consent for participation in the study was obtained either directly, or from a guardian of each patient. This study was approved by the Research Ethics Committee, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Full table
SNP selection
The preliminary screening criteria for ROCK1 gene polymorphisms were (I) minor allele frequency >5% in the Chinese Han population; (II) on the basis of previous literature data. This resulted in selection of three SNPs in ROCK1 (rs35996865, rs2127958, rs11873284) for inclusion in this study.
Blood samples, DNA isolation and genotyping
Genomic DNA was isolated from peripheral blood through standardized phenol/chloroform extraction. Three single nucleotide polymorphisms (rs35996865, rs2127958, rs11873284) and APOE were genotyped through PCR using flanking primers and Sanger sequencing. Primers of ROCK1 were designed according to the RefSeq gene sequence. Optimized primer sequences are listed in supplementary Table S1. Primers of APOE were used as described in Zivelin et al. (19). PCR conditions are available on request.

Full table
Statistical analysis
Results are expressed as mean ± SD or percentage as indicated. To compare the differences of mean values between two groups, unpaired Student’s t-test was used. Goodness-of-fit to the Hardy-Weinberg equilibrium (HWE) and differences in genotype and allele frequencies between the cases and controls were calculated by Chi-squared analysis with Fisher’s exact tests. The criterion for significance level was set at a P value of 0.01 for all tests. All probability values were based on two-tailed tests. SPSS was used for statistical analysis. Haplotype analysis was performed by using online software, SHEsis (http://analysis.bio-x.cn/myAnalysis.php), SNPStats (https://www.snpstats.net/snpstats/start.htm?q=snpstats/start.htm) was used to evaluate the association between SNPs and the risk of AD under 4 inheritance models, including codominant, overdominant, dominant, and recessive models.
Results
In the present study, a total of 295 patients with AD and 206 unrelated gender- and age-matched (P>0.05) controls were investigated. Table 1 shows the clinical features of the study population.
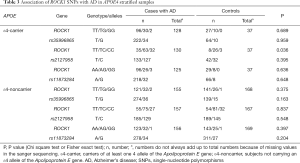
Table 2 shows the distribution of genotypes and alleles for ROCK1 and APOEε4 between the case and control. No significant deviations from HWE were found for ROCK1 in two groups. Our study demonstrated no significant differences between groups among three ROCK1 polymorphisms in allele frequencies and genotype distributions (Table 2). Further, the three SNP genotype frequencies and allele frequencies also did not show significant differences between patients of AD and controls in APOEε4-stratified subjects (Table 3) (P>0.01).

Full table
Full table
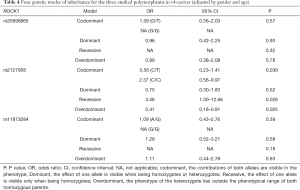
To further investigate the ROCK1 genetic association of AD, four-inheritance models were assumed and analysis was performed by logistic regression among ε4-carriers. The three SNPs did not show significant differences adopting any of the four-inheritance model (Table 4).
Full table
Discussion
To the best of our knowledge, this is the first study to investigate the contribution of ROCK1 gene variants to risk for AD. According to molecular neuropathogenic investigations distinct from GWAS, we put forward a novel hypothesis that ROCK1 variation could account for some risk of developing AD. Unfortunately, our outcomes do not show evidence of ROCK1 associations to AD risk among AD patients, even when stratified by APOE genotype. As the strongest genetic determinant of AD, APOEε4 allele associates with increased incidence of AD, whereas the ε2 allele decreases risk. Studies in humans and transgenic mice showed that APOE regulates Aβ metabolism, aggregation and clearance in the brain in an isoform-dependent manner (20-23). For example, APOEε4 astrocytes eliminate Aβ plaques less effectively than APOEε3 astrocytes (24), and clearance of Aβ by endocytosis is impaired by APOEε4 (25). Interestingly, Iizuka and coworkers found ROCK1 immunoreactivity was distributed in astrocytes and Bergmann glial cells that actually produce APOE in the CNS (26). Again, our previous study shows that ROCK1 colocalizes with highly fibrilized Aβ and that activated ROCK1 inhibits the autophagic clearance of Aβ (12). Both APOE and ROCK1 colocalize with plaque-associated amyloid and glial cells, suggesting a role for ROCK1 in pathogenesis of AD by interacting with APOE4. Follow-up functional studies are still needed to confirm these findings.
Interestingly, two studies have identified ROCK1 as a risk factor of brain diseases. Robert and colleagues investigated the role of ROCK1 gene variations played in ischemic stroke and found that there were obvious associations between ROCK1 gene variants (rs2127958, rs11873284) and ischemic stroke in Caucasian women (14). Evidence from clinical studies has shown that ROCK activity was elevated in patients with acute ischemic stroke (27) and inhibition of ROCK activity by statins probably helps to prevent ischemic stroke (28-30). Non-synonymous SNPs (rs111874856, rs112130712, rs112108028, rs73963110) are involved in the risk of Behcet’s disease in the Turkish population (15). The frequencies of these four variants are extremely rare to absent in Chinese populations according to the NCBI database. Both studies looked at Caucasians, which have a different genetic background from Asians. Additionally, the ROCK1 gene polymorphism rs35996865 mapping to the 5’-UTR was significantly associated with colorectal cancer (16), obesity-related metabolic syndrome (17), renal cell carcinoma (18) and respiratory distress syndrome (31), but not AD. Nonetheless, we have detected elevated ROCK1 activity in the peripheral blood of AD patients (unpublished data) and found that ROCK1 plays an important role in pathogenic mechanisms of AD. However, our association study did not show that any of the three SNPs examined increases the risk of AD.
Results from this study may have some implications for future work, in order to clarify the role for increasing AD risk of the APOEε4 allele in association with ROCK1 gene variants. The transcription level of the APOE gene should be considered with ROCK1 SNP status to determine their potential cooperative role in the progression of AD. Further, the combined effects of APOE4 and ROCK1 on senile plaque clearance and phagocytosis by astrocytes still remains to be examined. One of the limitations of this study is small sample size. Larger sample size and different ethnic groups would be helpful for explaining the involvement of ROCK1 in AD pathogenesis. Moreover, further analysis of the correlations of other polymorphisms in the ROCK1 gene and clinical manifestations is warranted in future.
In summary, we present here the first multicenter pilot study to evaluate the contribution of ROCK1 variants to AD risk. Our data demonstrated that the ROCK1 gene may not influence the risk of AD by interacting with APOE among Chinese Han people.
Acknowledgements
Funding: We thank the patients and their families for their participation in this project. We thank Dr. Eric B. Dammer at the Department of Biochemistry, Center for Neurodegenerative Diseases, Emory University School of Medicine for his critical reading. This study was supported by the National Natural Science Foundation of China (No. 81671043) and the “Shuguang Program (16SG15)” supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission. The authors declare no competing financial interests.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Research Ethics Committee, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China (No. 2016-127). Informed consent for participation in the study was obtained either directly, or from a guardian of each patient.
References
- Loy CT, Schofield PR, Turner AM, et al. Genetics of dementia. Lancet 2014;383:828-40. [Crossref] [PubMed]
- Borgaonkar DS, Schmidt LC, Martin SE, et al. Linkage of late-onset Alzheimer's disease with apolipoprotein E type 4 on chromosome 19. Lancet 1993;342:625. [Crossref] [PubMed]
- Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993;261:921-3. [Crossref] [PubMed]
- Scheltens P, Blennow K, Breteler MM, et al. Alzheimer's disease. Lancet 2016;388:505-17. [Crossref] [PubMed]
- Nakagawa O, Fujisawa K, Ishizaki T, et al. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett 1996;392:189-93. [Crossref] [PubMed]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 2003;4:446-56. [Crossref] [PubMed]
- Hensel N, Rademacher S, Claus P. Chatting with the neighbors: crosstalk between Rho-kinase (ROCK) and other signaling pathways for treatment of neurological disorders. Front Neurosci 2015;9:198. [Crossref] [PubMed]
- Zhou Y, Su Y, Li B, et al. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science 2003;302:1215-7. [Crossref] [PubMed]
- Tang BL, Liou YC. Novel modulators of amyloid-beta precursor protein processing. J Neurochem 2007;100:314-23. [Crossref] [PubMed]
- Henderson BW, Gentry EG, Rush T, et al. Rho-associated protein kinase 1 (ROCK1) is increased in Alzheimer's disease and ROCK1 depletion reduces amyloid-beta levels in brain. J Neurochem 2016;138:525-31. [Crossref] [PubMed]
- Hu YB, Dammer EB, Ren RJ, et al. The endosomal-lysosomal system: from acidification and cargo sorting to neurodegeneration. Transl Neurodegener 2015;4:18. [Crossref] [PubMed]
- Hu YB, Zou Y, Huang Y, et al. ROCK1 Is Associated with Alzheimer's Disease-Specific Plaques, as well as Enhances Autophagosome Formation But not Autophagic Abeta Clearance. Front Cell Neurosci 2016;10:253. [Crossref] [PubMed]
- Wang G, Huang Y, Wang LL, et al. MicroRNA-146a suppresses ROCK1 allowing hyperphosphorylation of tau in Alzheimer's disease. Sci Rep 2016;6:26697. [Crossref] [PubMed]
- Zee RY, Wang QM, Chasman DI, et al. Gene variations of ROCKs and risk of ischaemic stroke: the Women's Genome Health Study. Clin Sci (Lond) 2014;126:829-35. [Crossref] [PubMed]
- Oguz E, Demiryurek AT, Pehlivan Y, et al. Association of Rho-kinase 1 (ROCK1) gene polymorphisms with Behcet's disease. Mol Diagn Ther 2014;18:419-26. [Crossref] [PubMed]
- Sari I, Berberoglu B, Ozkara E, et al. Role of rho-kinase gene polymorphisms and protein expressions in colorectal cancer development. Pathobiology 2013;80:138-45. [Crossref] [PubMed]
- Tabur S, Oztuzcu S, Oguz E, et al. Association of Rho/Rho-kinase gene polymorphisms and expressions with obesity-related metabolic syndrome. Eur Rev Med Pharmacol Sci 2015;19:1680-8. [PubMed]
- Zhao R, Liu K, Huang Z, et al. Genetic Variants in Caveolin-1 and RhoA/ROCK1 Are Associated with Clear Cell Renal Cell Carcinoma Risk in a Chinese Population. PLoS One 2015;10:e0128771. [Crossref] [PubMed]
- Zivelin A, Rosenberg N, Peretz H, et al. Improved method for genotyping apolipoprotein E polymorphisms by a PCR-based assay simultaneously utilizing two distinct restriction enzymes. Clin Chem 1997;43:1657-9. [PubMed]
- Deane R, Sagare A, Hamm K, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 2008;118:4002-13. [Crossref] [PubMed]
- Jiang Q, Lee CY, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron 2008;58:681-93. [Crossref] [PubMed]
- Kok E, Haikonen S, Luoto T, et al. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol 2009;65:650-7. [Crossref] [PubMed]
- Schmechel DE, Saunders AM, Strittmatter WJ, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A 1993;90:9649-53. [Crossref] [PubMed]
- Simonovitch S, Schmukler E, Bespalko A, et al. Impaired Autophagy in APOE4 Astrocytes. J Alzheimers Dis 2016;51:915-27. [Crossref] [PubMed]
- Li J, Kanekiyo T, Shinohara M, et al. Differential regulation of amyloid-β endocytic trafficking and lysosomal degradation by apolipoprotein E isoforms. Journal of Biological Chemistry 2012;287:44593. [Crossref] [PubMed]
- Iizuka M, Kimura K, Wang S, et al. Distinct distribution and localization of Rho-kinase in mouse epithelial, muscle and neural tissues. Cell Struct Funct 2012;37:155-75. [Crossref] [PubMed]
- Feske SK, Sorond FA, Henderson GV, et al. Increased leukocyte ROCK activity in patients after acute ischemic stroke. Brain Res 2009;1257:89-93. [Crossref] [PubMed]
- Rawlings R, Nohria A, Liu PY, et al. Comparison of effects of rosuvastatin (10 mg) versus atorvastatin (40 mg) on rho kinase activity in caucasian men with a previous atherosclerotic event. Am J Cardiol 2009;103:437-41. [Crossref] [PubMed]
- Liu PY, Liu YW, Lin LJ, et al. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation 2009;119:131-8. [Crossref] [PubMed]
- Nohria A, Prsic A, Liu PY, et al. Statins inhibit Rho kinase activity in patients with atherosclerosis. Atherosclerosis 2009;205:517-21. [Crossref] [PubMed]
- Kaya G, Sivasli E, Oztuzcu S, et al. Association of Rho-kinase Gene Polymorphisms with Respiratory Distress Syndrome in Preterm Neonates. Pediatr Neonatol 2017;58:36-42. [Crossref] [PubMed]


