The uremic solute-AHR-tissue factor axis in vascular cells, mouse models and thrombosis in chronic kidney disease patients
Patients with chronic kidney disease (CKD) have an increased risk of cardiovascular disease and mortality (1). Patients with the lowest glomerular filtration rates have the higher risk (2). Nonclassical risk factors, such as inflammation and endothelial dysfunction, appear to drive cardiovascular disease in these patients (3,4). In addition, CKD patients exhibit a prothrombotic state with increased levels of procoagulant factors (factor VII, factor VIII and plasminogen activator inhibitor type-1) and markers of activation of coagulation (prothrombin 1+2 and thrombin-antithrombin complexes) (5-7). CKD patients also have an increase in tissue factor (TF) expression on circulating monocytes (8). TF is the cellular initiator of the coagulation cascade and contributes to thrombosis associated with a variety of diseases (9). Importantly, epidemiology studies showed that CKD patients have an increased risk of venous thromboembolism (VTE) (deep venous thrombosis and pulmonary embolism) (10-12).
The reduction in glomerular filtration rates in CKD patients leads to uremia with the accumulation of metabolic breakdown products of tryptophan from the indolic and kynurenine pathways. Accumulation of these toxic products is further accelerated by the loss of organic anion transporters (OATs) associated with destruction of nephrons. Oat1 and Oat3 are the main transporters expressed in the proximal tubule of the nephron that excrete a variety of potential toxins, including indolic and kynurenine pathway products (13). Accumulation of these products likely contributes to inflammation and vascular dysfunction.
Importantly, TF is not expressed by unstimulated ECs and peripheral blood mononuclear cells (PBMCs), whereas VSMCs constitutively express TF (9). An early study showed that uremic serum induced TF mRNA and protein expression in human endothelial cells (ECs) (14). In 2013, two papers reported that the uremic solutes indoxyl sulfate (IS) and indole-3-acetic acid (IAA) increased TF expression in human ECs, PBMCs and vascular smooth muscle cells (VSMCs) (15,16).
Gondouin and colleagues showed that IS and IAA induced the transient expression of TF mRNA and protein in human umbilical vein ECs (HUVECs) and PBMCs, and the release of TF-positive microvesicles from HUVECs (15). This induction was mediated by activation of the transcription factor aryl hydrocarbon receptor (AHR) (Figure 1A) (15). Induction of TF expression was abolished by reducing AHR expression using a small interfering RNA and by the AHR inhibitor geldanamycin. In addition, the AHR agonist 2,3,7,8-tetrachlorodiebenzo-p-dioxin induced TF expression in HUVECs. Since the TF promoter does not contain a consensus AHR binding site (XRE) the authors proposed that AHR induced TF gene expression via either a noncanonical XRE, by interacting with other transcription factors, such as NF-κB, or by enhancing a signaling pathway that regulates TF gene expression (15). TF expression by ECs and PBMCs may contribute to the increased incidence of thrombotic events in CKD patients.
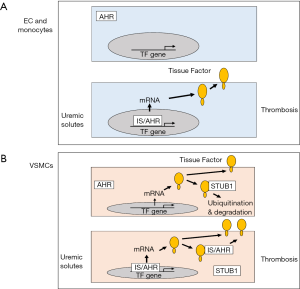
Chitalia and colleagues focused on human VSMCs because they expressed higher levels of TF than human coronary artery EC and a human monocytic leukemia cell line THP-1 (16). Surprisingly, it was reported that uremic serum did not induce TF mRNA expression in VSMCs. However, exposure of VSMCs to uremic serum, IS or IAA for 24 hours increased TF protein expression by prolonging its half-life. Since the ubiquitination and proteasomal degradation pathway plays a central role in regulating protein stability, they determined if this pathway regulated TF stability. Indeed, baseline ubiquitination of TF in human VSMCs was observed when the cells were treated with a proteasome inhibitor (16). IS and IAA inhibited TF ubiquitination which resulted in an increase in its half-life and an increase in TF protein on the surface of cells (Figure 1B).
In a second paper, Chitalia and colleagues showed that IS increased TF protein expression in human aortic VSMCs in an AHR-dependent manner (17), which is consistent with the work of Gondouin and colleagues with ECs and PBMCs (15). In this study, IS increased TF mRNA expression in VSMCs by 10-fold after 4 hours. It is unclear why IS induction of TF mRNA was not observed in the earlier study (16). Increased TF protein expression in VSMCs exposed to either uremic serum or IS was abolished by two different AHR antagonists. Furthermore, AHR and TF could be co-immunoprecipitated and the purified proteins directly interacted (17). This study suggested that AHR may also increase TF protein expression via a non-genomic mechanism (see below).
The third paper by Chitalia and colleagues reported that the ubiquitin ligase STIP1 homology and U-box-containing protein 1 (STUB1) [also known as carboxyl terminus of hsc70-interacting protein (CHIP)] bound to TF and directed its degradation via the proteasome pathway (Figure 1B) (18). Silencing of STUB1 in primary aortic VSMCs was associated with a significant increase in TF protein under both uremic and non-uremic conditions. Similarly, silencing of STUB1 also increased TF protein expression in HUVECs presumably by increasing its stability. TF contains a short cytoplasmic domain and deletion of this domain abolished STUB1 binding suggesting that it contains a binding site for STUB1 (18). As expected, IS reduced the interaction between TF and STUB1 consistent with a model in which IS inhibits TF ubiquitination and increases its stability. Moreover, addition of an AHR antagonist to IS treated cells restored binding of STUB1 to TF. Since AHR has been shown to bind to TF we postulate that liganded AHR may bind to TF and displace STUB1 resulting in stabilization of the protein (Figure 1B). This model needs to be tested.
Mouse models of CKD can be used to study the pathways that contribute to thrombosis. The 5/6 nephrectomy has a high rate of mortality and has a relatively large inter-mouse variation. Surprisingly, a prolonged rather than a shortened occlusion time was observed in a photochemical induced carotid artery thrombosis model in a 5/6 nephrectomy mouse model of CKD (19). An alternative model of CKD is the adenine diet model. Adenine is converted to 2,8-dihydroxyadenine by xanthine dehydrogenase and this precipitates in the kidney tubules leading to nephrolithiasis and renal dysfunction (20). One study found that a casein-based diet blunted the smell and taste of adenine and made it more palatable (20). Mice fed the adenine diet for 8 weeks had a significant increase in blood urea (80–100 mg/mL), a doubling of plasma creatine and tubulointerstitial damage with infiltration of leukocytes (20).
Chitalia and colleagues chose to established a new solute-specific mouse model with elevated levels of IS in the plasma (18). This model involved administration of IS in water together with the twice daily injection of probenecid, which inhibits OATs and IS excretion. Plasma levels of IS increased to ~55 and ~75 µg/mL at 2 and 5 days, respectively. However, it should be noted that this is not a model of CKD but rather a model of acute exposure to a uremic solute. Mice with elevated IS (IS mice) exhibited a significantly shortened time to occlusion in the ferric chloride carotid artery thrombosis model. Importantly, the AHR antagonist CH223191 increased the occlusion time in IS mice to the baseline of control mice (18). Similar results were observed by Chitalia and colleagues using a second uremic solute model that used L-kynurenine in place of IS (21). The Shashar paper (18) also presented data with an adenine diet model. Mice were fed the diet for 2 weeks, which is considerably shorter than the 8 weeks reported in the paper describing this model (20). There was no data presented on tubulointerstitial damage or infiltration of leukocytes. Nevertheless, blood urea increased to ~100 mg/mL and plasma IS increased to ~15 µg/mL, which is considerably lower than the level observed with administration of IS. Mice fed the adenine diet had a shortened time to occlusion in the ferric chloride carotid artery thrombosis model compared with controls and this was reversed with either CH223191.
Finally, an anticancer agent was used to increase levels of STUB1. The compound 2-(4-hydroxy-3-methoxyphenyl)-benzothiazole (YL-109) was shown to increase STUB1 expression in the human breast cancer line MDA-MD-231 via AHR (22). This is rather odd since an AHR agonist would also be expected to increase TF expression. Nevertheless, YL-109 increased STUB1 and decreased TF expression in the aortas of mice injected with IS (18). YL-109 also increased the occlusion time in the ferric chloride carotid arterys thrombosis model in mice given IS or fed the adenine diet.
Inhibition of TF with a rat anti-mouse monoclonal antibody also prolonged the occlusion time in CKD mice. However, the antibody inhibits all sources of TF so this result simply shows that TF is involved in thrombosis in the ferric chloride carotid artery thrombosis model. YL-109 decreased the level of TF expression in the aortas of mice injected with IS. However, no controls were presented so it is unclear if IS increased TF expression in the aorta. Moreover, it would have been very helpful to know the levels of TF in the carotid arteries of control mice and also mice with elevated levels of IS or fed the adenine diet for 5 days. This would support the notion that the shortened occlusion time in the ferric chloride thrombosis model is due to increased levels of TF expression in the vessel wall.
We have shown that deletion of TF in VSMCs is associated with a significantly prolonged occlusion time in the ferric chloride carotid artery thrombosis model (23). Since Chitalia and colleagues have focused on TF expression in VSMCs, it would be interesting to know if deletion of TF in these cells abolishes the shortened occlusion times observed in mice with elevated levels of IS or fed the adenine diet.
There are a variety of mouse models of thrombosis that can be divided into arterial and venous models (24). The ferric chloride and photochemical induced carotid artery models are the most common arterial models but are associated with denudation of the endothelium. It would be interesting to see if there is increased thrombosis in venous models, such as the infrarenal stenosis and stasis models, in mice with elevated IS or fed the adenine diet. The role of myeloid TF and EC TF in any enhanced thrombosis could be evaluated using mice with cell type-specific deletion of TF (25). These studies may reveal roles for TF expression in ECs and/or leukocytes in thrombosis in the solute-specific and CKD models.
The identification of the uremic solute-AHR-TF axis is an exciting advance in our knowledge of possible mechanisms of thrombosis in CKD patients. AHR antagonists may represent a novel approach to reduce the risk of thrombosis in these patients.
Acknowledgements
We would like to acknowledge Drs. Antoniak, Grover and Schisler for helpful comments.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vanholder R, Massy Z, Argiles A, et al. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 2005;20:1048-56. [Crossref] [PubMed]
- Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010;375:2073-81. [Crossref] [PubMed]
- Stam F, van Guldener C, Schalkwijk CG, et al. Impaired renal function is associated with markers of endothelial dysfunction and increased inflammatory activity. Nephrol Dial Transplant 2003;18:892-8. [Crossref] [PubMed]
- Panichi V, Rizza GM, Paoletti S, et al. Chronic inflammation and mortality in haemodialysis: Effect of different renal replacement therapies. Results from the riscavid study. Nephrol Dial Transplant 2008;23:2337-43. [Crossref] [PubMed]
- Baskin E, Duman O, Beşbaş N, et al. Hypercoagulopathy in a hemodialysis patient: Are elevations in factors VII and VIII effective? Nephron 1999;83:180. [Crossref] [PubMed]
- Segarra A, Chacón P, Martinez-Eyarre C, et al. Circulating levels of plasminogen activator inhibitor type-1, tissue plasminogen activator, and thrombomodulin in hemodialysis patients: Biochemical correlations and role as independent predictors of coronary artery stenosis. J Am Soc Nephrol 2001;12:1255-63. [PubMed]
- Malyszko J, Malyszko JS, Mysliwiec M. Comparison of hemostatic disturbances between patients on capd and patients on hemodialysis. Perit Dial Int 2001;21:158-65. [PubMed]
- Mercier E, Branger B, Vecina F, et al. Tissue factor coagulation pathway and blood cells activation state in renal insufficiency. Hematol J 2001;2:18-25. [Crossref] [PubMed]
- Grover SP, Mackman N. Tissue factor: An essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol 2018;38:709-25. [Crossref] [PubMed]
- Wattanakit K, Cushman M, Stehman-Breen C, et al. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol 2008;19:135-40. [Crossref] [PubMed]
- Kayali F, Najjar R, Aswad F, et al. Venous thromboembolism in patients hospitalized with nephrotic syndrome. Am J Med 2008;121:226-30. [Crossref] [PubMed]
- Mahmoodi BK, Gansevoort RT, Næss IA, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: Pooled analysis of five prospective general population cohorts. Circulation 2012;126:1964-71. [Crossref] [PubMed]
- Wu W, Bush KT, Nigam SK. Key role for the organic anion transporters, oat1 and oat3, in the in vivo handling of uremic toxins and solutes. Sci Rep 2017;7:4939. [Crossref] [PubMed]
- Serradell M, Díaz-Ricart M, Cases A, et al. Uremic medium disturbs the hemostatic balance of cultured human endothelial cells. Thromb Haemost 2001;86:1099-105. [Crossref] [PubMed]
- Gondouin B, Cerini C, Dou L, et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int 2013;84:733-44. [Crossref] [PubMed]
- Chitalia VC, Shivanna S, Martorell J, et al. Uremic serum and solutes increase post-vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation 2013;127:365-76. [Crossref] [PubMed]
- Shivanna S, Kolandaivelu K, Shashar M, et al. The aryl hydrocarbon receptor is a critical regulator of tissue factor stability and an antithrombotic target in uremia. J Am Soc Nephrol 2016;27:189-201. [Crossref] [PubMed]
- Shashar M, Belghasem ME, Matsuura S, et al. Targeting stub1-tissue factor axis normalizes hyperthrombotic uremic phenotype without increasing bleeding risk. Sci Transl Med 2017.9. [PubMed]
- Kokubo T, Ishikawa N, Uchida H, et al. Ckd accelerates development of neointimal hyperplasia in arteriovenous fistulas. J Am Soc Nephrol 2009;20:1236-45. [Crossref] [PubMed]
- Jia T, Olauson H, Lindberg K, et al. A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrology 2013;14:116. [Crossref] [PubMed]
- Kolachalama VB, Shashar M, Alousi F, et al. Uremic solute-aryl hydrocarbon receptor-tissue factor axis associates with thrombosis after vascular injury in humans. J Am Soc Nephrol 2018;29:1063-72. [PubMed]
- Hiyoshi H, Goto N, Tsuchiya M, et al. 2-(4-hydroxy-3-methoxyphenyl)-benzothiazole suppresses tumor progression and metastatic potential of breast cancer cells by inducing ubiquitin ligase chip. Sci Rep 2014;4:7095. [Crossref] [PubMed]
- Wang L, Miller C, Swarthout RF, et al. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood 2009;113:705-13. [Crossref] [PubMed]
- Jagadeeswaran P, Cooley BC, Gross PL, et al. Animal models of thrombosis from zebrafish to nonhuman primates: Use in the elucidation of new pathologic pathways and the development of antithrombotic drugs. Circ Res 2016;118:1363-79. [Crossref] [PubMed]
- Pawlinski R, Wang JG, Owens AP 3rd, et al. Hematopoietic and nonhematopoeitc cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood 2010;116:806-14. [Crossref] [PubMed]


