The role of epithelial growth factors and insulin growth factors in the adrenal neoplasms
Introduction
Insulin-like growth factors (IGFs) are proteins with high sequence similarity to insulin and are part of a complex signaling system playing a critical role in normal growth and development as well as in tumorigenesis (1). The IGF system is comprised by the IGF ligands (IGF-1, IGF-2, insulin), their cell surface receptors [IGF-1R, IGF-2R, and insulin receptor (IR)], that mediate the effects of the IGFs, the family of six high-affinity IGF-binding proteins (IGFBP-1, -2, -3, -4, -5, -6) which modulated IGFs action and bioavailability as well as the IGFBP degrading enzymes (proteases) (2-4). IGF-2 gene is located in chromosome 11 (11p15.5) and encodes IGF-2 protein which stimulates cell survival and proliferation via binding to the IGF-1R. IGF-1 and IGF-2 share ~65% and ~50% homology with insulin molecule, respectively (5,6).
IGF-1 is located in chromosome 12 (12q22–24.1) and several studies suggest that high levels of circulating IGF-1 constitute a risk factor for the development of breast, prostate, colon, and lung cancer. The role of IGF1-R has been extensively studied in tumorigenesis of endocrine malignancies such as thyroid and adrenal cancer (1,4). Upon ligand binding, the intrinsic tyrosine kinases of both IR and IGF1-R are activated leading to a sequential activation of two main downstream pathways, the mitogen-activated protein kinases cascade (MAPKs) and the PI3K pathway (4,7). Both MAPK and PI3K pathways enhance proliferation and cell growth through mTOR activation (Figure 1).
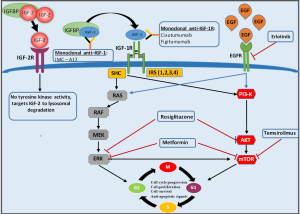
Epithelial growth factor (EGF) gene is located in chromosome 7 (7p12) and encodes a protein that acts through binding with high affinity to cell surface EGF-R. EGF-R is a tyrosine kinase receptor which belongs to the ErbB family (5). Members of this receptor family play important role in various biologic responses such as proliferation, differentiation, cell motility, and survival through binding of the extracellular growth factor ligands and activation of intracellular signaling pathways (3,4). EGF-R upon ligand stimulation, activates two major intracellular pathways: the MAPK pathway which controls gene transcription, cell-cycle progression from the G1 phase to the S phase as well as cell proliferation, and the PI3K-Akt pathway, which activates a cascade of anti-apoptotic and survival signals (8) (Figure 1).
EGF-R signaling found to be dysregulated as a result of either point mutations occurring in exons 18–21 of EGFR, corresponding to the tyrosine kinase (TK) domain, or chromosome 7 polysomy. In both cases, EGFR protein is constitutively activated in a ligand independent manner, leading, in turn, to the activation of downstream signal transduction pathways, mainly represented by the Ras/Raf/MEK/ERK and the PI3K/PTEN/AKT pathways. This activation leads to tumour growth, metastasis, angiogenesis and inhibition of apoptosis (9).
Herein, we aim to present recent data on the implication of IGF and EGF pathways in the adrenal development and tumorigenesis as well as their role as a potential treatment of adrenocortical carcinoma (ACC) a rare malignancy with an estimated annual incidence of 0.7–2 cases by year and a global prevalence of 4–12 cases per million and very poor prognosis (5-year survival rate inferior to 35% in most series) (10).
Role of IGF and EGF in adrenal adenomas and hyperplasia
Adrenocortical tumorigenesis, similarly to oncogenesis in other tissues, is a multi-step process associated with an increasing number of genetic alterations (11,12). Although these alterations are rather rare in hyperplasias and small adenomas, in some cases, they might correlate to the development of benign adrenal tumors. Monoclonal expansion is the most important genetic feature that differentiates adrenocortical adenomas (ACAs) from hyperplasias which is a polyclonal process, indicating that genetic changes at specific loci in the genome are needed for adrenal tumorigenesis (11).
A number of chromosomal abnormalities have been implicated in the tumorigenesis of the adrenal cortex, including genomic loci on 11p and 17p (13) which harbor oncosuppressive genes coding for the p53 (on 17p13.1) (14), and the p57 (on 11p15.5) (KIP2) (15) proteins and others such as the IGF-2 (on 11p15.5) protein (16). It is well known that p53 mutations are more frequent in ACC, however they are also rarely reported in large adenomas, as either chromosomal deletions or gains, where they represent a sentinel event of the Li-Fraumeni syndrome—a rare disorder that greatly increases the risk of developing several types of cancer, particularly in children and young adults including breast cancer, sarcomas and ACC, or occur as low penetrance germline mutation outside of the context of a particular syndrome (17). Somatic allelic loss of the 17q22–24 region has also been found in sporadic adrenocortical tumors (23% of adenomas and 53% of ACCs), especially those presenting with Cushing syndrome (17). However, none of above-mentioned genes appears to be specific for adrenocortical tumor pathogenesis (18).
Normal adult and fetal human adrenal gland express both types of IGF-R and a variety of specific IGFBPs in addition to IGF-1 and IGF-2, suggesting their potential role in the regulation of adrenal growth and function (19,20). Of note, both IGF-Rs and the IR are expressed at a similar level in all three zones of the adrenal cortex (21). Thus, IGF-R expression is considered mainly as a tumor progression marker rather than an initiating factor of tumorigenesis (22,23).
Both IGFs stimulate basal and ACTH-induced steroid biosynthesis by upregulating steroidogenic key enzymes and ACTH-receptor expression (20). Additionally, IGF-1 and IGF-2 preferentially stimulate adrenal androgen secretion through IGF-1R. In human adult adrenocortical cells, the expression and secretion of IGFBPs are differentially regulated by ACTH, steroid hormones and IGFs (24).
IGF-2 overexpression is essential for the growth of monoclonal lesions, such as large adenomas and ACC (25) and is thought to contribute to tumorigenesis in Beckwith-Wiedemann Syndrome, an overgrowth disorder usually present at birth, characterized by an increased risk of childhood cancer particularly Wilms’ tumor (nephroblastoma), hepatoblastoma and ACCs (26). Overexpression of IGF-2 can be caused by paternal isodisomy (maternal allele is normally silenced), in which the maternal allele is lost and the paternal allele is duplicated, or by maternal inheritance of microdeletions of the imprinting center such that the maternal allele is no longer silenced (26,27). Although, IGF-2 was overexpressed in both ACC and ACA in a pediatric population, the expression of IGF1-R, which mediates IGF-2 effects in vivo, was upregulated in pediatric ACCs compared to pediatric ACAs although its expression was similar to both adult ACCs and ACAs (28).
It is well-known that normal function of both adrenocorticotropin (ACTH) and its receptor (termed the melanocortin 2 receptor) is crucial for the adrenocortical growth, differentiation and function. On the other hand, ACTH action is mediated by the cyclic AMP (cAMP) signaling pathway. An overactivated cAMP signaling pathway is involved in most adrenocortical hyperplasias and occasionally in sporadic adenomas (25,29). Micronodular bilateral adrenocortical hyperplasia and its more well-known variant, primary pigmented nodular adrenocortical disease (PPNAD), are correlated with germline-inactivating mutations of the PRKAR1A gene which encodes the cAMP-dependent protein kinase (PKA) type I-alpha regulatory subunit (30,31). In vitro and in vivo studies have shown that both IGFs as well as IGFBP-2 and -3 expression were increased in PPNAD caused by PRKAR1A mutations compared to those without the mutation, implying that PRKAR1A gene alterations are associated with a dysregulation of IGF axis. Moreover, PKA inhibitors increased IGFBP-2 expression in NCI-H295R adrenocortical cells, while anti-IGFBP-2 antibody reduced their proliferation (32). However, IGFBP-2 blood levels were found similar to both healthy controls and patients with adrenal adenomas whereas they were increased in ACC (33).
There are only few data published on IGFs axis and congenital adrenal hyperplasia (CAH). Pubertal rise of total serum IGF-1 is earlier in children with classic CAH due to 21-OH deficiency (CAH), whereas the molar ratio of IGF-1 to IGFBP-3 is normal at pre-pubertal ages but decreased in puberty. Serum IGFBP-3 levels are elevated at all ages but lower in pubertal children. Although the IGF-1: IGFBP-3 molar ratio has been suggested to reflect bioactive IGF-1, the fact that most IGFBP-3 binding sites in the circulation are occupied by IGF-2 and not by IGF-1remains a significant limitation (34).
Studies in IGF-1/IGF-1R mRNA expression and immunostaining in human adrenals showed very low IGF-1/IGF-1R expression in the zona reticularis, from early infancy to late puberty, suggesting that the IGF system is not directly involved in the regulation of adrenal androgen synthesis and secretion (35). Therefore, it has been proposed that IGF-1 and perhaps IGF-2 are involved at another level, either by autocrine, paracrine, or endocrine stimulation, in the postnatal mechanisms of progenitor adrenal cell proliferation and migration (36).
Adrenal lesions such as adenoma/hyperplasia detectable by CT were found more frequently in acromegalic patients than in the general population, with a prevalence of 28.7%, although no single factor (GH/IGF-2 levels or disease duration) predicts them. Interestingly, patients with and without adrenal lesions exhibit no differences concerning GH, IGF-2 levels and duration of disease (37).
In vitro experiments in fetal human adrenal cells have showed that the EGF plays important role in the development of the adrenal gland by stimulating their proliferation. EGF also stimulates the secretion of cortisol in sheep adrenal gland (38). However, data on the role of EGF and EGF-R in human adrenocortical tumorigenesis are conflicting. Several studies have reported EGF-R overexpression in ACCs but not in ACA, implying that EGFR could potentially be used as a marker for the differential diagnosis of ACAs and ACCs (39,40). In particular, EGF-R exhibited no expression in normal adrenal tissues whereas it was hardly detectable in ACAs—either functional or nonfunctional—of maximum diameter less than 3 cm, indicating that the degree of its expression in adrenocortical tumors may be positively correlated with a more malignant phenotype, and this could be used in the differential diagnosis between ACAs and ACCs (40).
Role of IGF and EGF in ACC
Somatic IGF-2 over-expression is one of the first and the most frequent molecular abnormalities described in sporadic adult ACC, with a very high prevalence of about 90%. In most of cases, this overexpression has been associated with DNA demethylation at IGF-2 locus, and paternal isodisomy (23,41) albeit not with tumor behavior and clinical prognosis (42). Because of the high prevalence of IGF-2 overexpression, it could not be considered as a marker for the ACC classification as either IGF-2-high or IGF-2-low tumors (43).
In vitro studies have demonstrated that IGF-2 and IGFBP-2 levels were increased in H295R ACC cells (44). However, studies in mouse models have showed that IGF-2 overexpression is not sufficient to promote tumorigenesis, even in association with beta-catenin activation suggesting that overexpression of IGF-2 might not be a driver force for adrenal tumorigenesis but simply the reflection of other genetic alterations at the 11p15.5 locus (45,46).
Dysregulation of noncoding RNA H19 was described in various types of cancers, including Bcr-Abl-induced leukemia (47). H19 promoter was shown to be highly methylated in ACC, leading to a decrease in H19 expression, suggesting a role of H19 in adrenal tumorigenesis (48).
ERK phosphorylation is essential step toward cell proliferation in many cancer tissues (49). The IGF-1 induced ERK1/2 and AKT pathway activation in ACC cells has been evaluated by various studies (50,51) (Figure 1). Genetic alterations such as RAS activating mutation (52) and BRAF mutations may negatively regulate the ERK1/2 pathway and lead to loss of the oncosuppressive PTEN gene expression in ACCs (53,54).
A recent study performed in H295R ACC cells has demonstrated an interaction between angiotensin II and insulin/IGF-1 axis applying a synergistic effect on ERK1/2 phosphorylation which might be responsible for elevated steroid hormones production observed in ACC patients (55). It has been shown that IGF-2 stimulates estrogen (E2) production in H295 ACC cells through triggering of steroidogenic factor-1 (SF-1) expression and aromatase activity. Elevated levels of estrogen lead to activation of the non-genomic signaling pathway of the estrogen receptor (ERα) resulting in ligand independent phosphorylation/activation of IGF-1R. This in turn triggers the phosphorylation of cAMP-responsive element bindings (CREB) protein leading to elevated IGF-1R protein levels. Additionally, both IGF-2 and IGF-1R can increase ERα phosphorylation through the activation of PI3K/ERK pathway leading to increased expression of CCND-1 gene and subsequent increased cell proliferation (Figure 2) (56). E2 induced cell proliferation is attenuated by Leucine-Rich protein-1 (FELP-1) silencing. It seems that FELP-1 controls ACC cell proliferation through coupling IGF-1R and ERα which is turn leads to activation of PELP1/ER/IGF-1R/c-Src pathway (57).
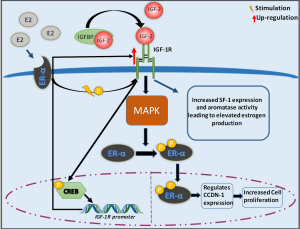
CYP19—a cytochrome P450 aromatase—is a key enzyme of estrogen biosynthesis which is not expressed in normal adrenal cortex. However, it is detectable in ACCs indicating a possible association between adrenal tumorigenesis and CYP19 expression. Incubation of H295R cells with EGF led to increased aromatase gene expression as well as aromatase activity through stimulation of prostaglandin E2 (PGE2) secretion and cAMP/PKA pathway (58). It was also demonstrated that EGF increased cortisol production in H295R ACC cells via up regulation of the 3β-hydroxysteroid dehydrogenase expression and activation of Stat5 gene (59) as well as androgen production via up-regulation of 17B-hydroxylase/lyase P450 (CYP17) expression in a MAPK/PKC independent pathway (60).
miR-483 found to be significantly overexpressed in pediatric ACCs while miR-99a and miR-100 was down-regulated in ACC (61-63). miR-483 is located in an intron of IGF-2 and its overexpression has been hypothesized to be the cause of IGF-2 locus dysregulation (61,62). Moreover, miR-99a and miR-100 target several components of the IGF-1 signaling pathway. Doghman et al. suggested that IGF-2/mTOR signaling activation in adrenal tumors is regulated by miR-99α and/or miR100 in multiple levels (63).
EGF-R overexpression has been reported in several cancer tissues such as non-small-cell lung cancer, head and neck cancer, pancreatic cancer, breast cancer and in more than 50% of ACCs (64-66). On the contrary EGF is not always detectable in these cancer tissues suggesting that the presence of other EGF-R specific ligands rather than EGF are necessary for the activation of EGF-R downstream signaling pathways (67,68). In vitro studies have shown that EGF-R levels in ACC tissues have also been positively associated with tumor growth and/or metastases (69).
Therapeutic implication of IGF and EGF inhibitors in the treatment of ACCs
Metformin and rosiglitazone are known as anti-diabetic drugs. It has been demonstrated that although rosiglitazone has no effect on IGF-1R level (70), metformin reduces IGF-2 and IGF-1R expression leading to inhibition of ACC cell proliferation (51,70). It appears that rosiglitazone exerts its anti-proliferative effects via inhibition of both ERK1/2 and AKT phosphorylation while metformin reduces ERK1/2 phosphorylation as well as mTOR activation (51,70).
IGF-1R kinase inhibitor (NVP-AEW541) found to exert anti-tumor effects in both adult and pediatric ACC cells lines (71). However, phase I trials evaluating the feasibility of IGF1-R (figitumumab) and/or IR inhibition (OSI-906-linsitinib) were quite disappointing as only one out of 25 patients showed a partial response according to RECIST criteria (45,72) (Table 1). Another phase I trial investigating linsitinib reported low efficacy, since only 2 out of 15 patients with ACC had a partial response to therapy (73,74). Furthermore, a larger phase III study including139 patients with advanced ACC treated with linsitinib, aiming to evaluate the overall survival, was discontinued prematurely because of drug failure (75) (Table 1).
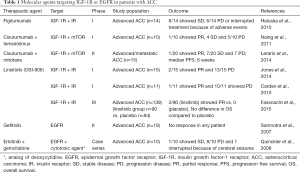
Full table
Recent in vitro experimental data indicated that IGF-1R inhibition combined with liposomal doxorubicin could represent a promising therapeutic approach for a subgroup of ACC patients with high levels of IGF-1R or for patients with high IGF-1R and IGF-2 level (76). Moreover, a phase I clinical trial including 10 ACC patients treated with a combination of monoclonal IGF-1R antibody (cixutumumab) and mTOR inhibitor (temsirolimus) showed that 4 out of these patients achieved disease stabilization (77). However, the efficacy of the combination of cixutumumab with mitotane in patients with advanced/metastatic ACC released disappointing results (78).
In vitro and animal studies also showed that proliferation of ACC cultured cells (H295R, SW-13, HAC15) and primary cells isolated from a pediatric ACT and ACC xenograft tumors were reduced by using everolimus, an IGF-2/mTOR pathway inhibitor (63). It has also been demonstrated that NVP-AEW541 and IMC-A12 (human IGF-1 monoclonal antibody) inhibited cell proliferation in both cultured cells (H295R and RL251) and ACC xenograft tumors (79,80).
EGF-R-targeted inhibitors such as tyrosine kinase inhibitors (TKIs) and anti-EGFR monoclonal antibodies have been tried in several in vitro studies with encouraging results. It has been shown that sunitinib, a well-known multiple TKI, reduced adrenocortical H295R and SW13 carcinoma cells viability acting through EGF-R-induced inhibition of ERK1/2 phosphorylation. Furthermore, erlotinib an EGF-R specific inhibitor decreased both H295R and SW13 cell viability, although SW13 cell line was more sensitive to erlotinib-induced apoptosis compared to H295R cells. Erlotinib induced cell apoptosis by blocking AKT phosphorylation and reduced cell viability by inhibition of MEK/ERK phosphorylation (68). The different response of the two afore-mentioned cell lines to erlotinib treatment was attributed to the difference of the basal and the phosphorylated levels of EGF-R (81). More importantly, the combination of erlotinib and NVP-AEW541 enhanced the anti-tumour efficacy compared to treatment with either agent alone or to untreated control, in both in vitro and animal experimentation (68). However, in humans, the data appears to be disappointed (Table 1). A phase II clinical trial including 19 patients with advanced ACC showed no significant response to gefitinib (EGFR/TKI) therapy (80). The combination of erlotinib and gemcitabine (cytotoxic agent) was tried for the treatment of 10 patients with advanced ACC with disappointing results as just 1 patient showed a progression-free survival of 8 months. Interestingly, the patients with lowest EGF-R expression at tissue level, showed the best response to the therapy (82).
Conclusions
IGFs and EGF axes are involved in the proliferation of the fetal human adrenal cells as well as in the pathogenesis of adrenal tumorigenesis and consist prognostic markers and therapeutic targets. IGF-2 over-expression is one of the first molecular abnormalities which has been described in sporadic adult ACC, with high prevalence of ~90%. However, no significant differences in clinical, biological and transcriptomic characteristics are found between IGF-2-high and IGF-2-low expressed tumors. Recent experimental data indicated that the combination of IGF-1R inhibition with liposomal doxorubicin may represent a promising therapeutic strategy for a subgroup of ACC patients with high levels of IGF-1R.EGF-R is over-expressed in ACCs compared to ACAs and associated with tumor growth and/or metastases as well as cortisol and androgen hypersecretion by adrenal tumor. IGF-1R and EGF-R kinase inhibitors have been shown to exert antitumor effects in vitro. Despite promising results in pre-clinical studies, using IGF-1R or EGF-R inhibitors alone or in combination therapy has not yet led to the expected therapeutic results in humans. However, the small number of the patients included in clinical trials (the majority were case series) as well as the advanced stage of ACC (all patients were metastatic already heavily treated and refractory in previous chemotherapies) could underestimate the therapeutic role of these agents which were used mostly as salvage therapies. Co-targeting the IGF-1R and EGF-R pathway at earlier stages of the disease and/or in combination with other antineoplastic drugs (cytotoxic drugs, mTOR inhibitors, monoclonal antibodies) in large clinical trials could be of great interest.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kasprzak A, Kwasniewski W, Adamek A, et al. Insulin-like growth factor (IGF) axis in cancerogenesis. Mutat Res Rev Mutat Res 2017;772:78-104. [Crossref] [PubMed]
- Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev 1989;10:68-91. [Crossref] [PubMed]
- Gallagher EJ, LeRoith D. Minireview: IGF, Insulin, and Cancer. Endocrinology 2011;152:2546-51. [Crossref] [PubMed]
- LeRoith D, Werner H, Beitner-Johnson D, et al. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev 1995;16:143-63. [Crossref] [PubMed]
- Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab 2000;278:E967-76. [Crossref] [PubMed]
- Philippou A, Christopoulos PF, Koutsilieris DM. Clinical studies in humans targeting the various components of the IGF system show lack of efficacy in the treatment of cancer. Mutat Res Rev Mutat Res 2017;772:105-22. [Crossref] [PubMed]
- Morcavallo A, Stefanello M, Iozzo RV, et al. Ligand-mediated endocytosis and trafficking of the insulin-like growth factor receptor I and insulin receptor modulate receptor function. Front Endocrinol (Lausanne) 2014;5:220. [Crossref] [PubMed]
- Riesco A, Santos-Buitrago B, De Las Rivas J, et al. Epidermal Growth Factor Signaling towards Proliferation: Modeling and Logic Inference Using Forward and Backward Search. Biomed Res Int 2017;2017. [Crossref] [PubMed]
- Martin V, Mazzucchelli L, Frattini M. An overview of the epidermal growth factor receptor fluorescence in situ hybridisation challenge in tumour pathology. J Clin Pathol 2009;62:314-24. [Crossref] [PubMed]
- Berruti A, Baudin E, Gelderblom H, et al. Adrenal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii131-8. [Crossref] [PubMed]
- Beuschlein F, Reincke M, Karl M, et al. Clonal composition of human adrenocortical neoplasms. Cancer Res 1994;54:4927-32. [PubMed]
- Bonnet-Serrano F, Bertherat J. Genetics of tumors of the adrenal cortex. Endocr Relat Cancer 2018;25:R131-52. [Crossref] [PubMed]
- Beckers A, Abs R, Willems PJ, et al. Aldosterone-secreting adrenal adenoma as part of multiple endocrine neoplasia type 1 (MEN1): loss of heterozygosity for polymorphic chromosome 11 deoxyribonucleic acid markers, including the MEN1 locus. J Clin Endocrinol Metab 1992;75:564-70. [PubMed]
- Lin SR, Lee YJ, Tsai JH. Mutations of the p53 gene in human functional adrenal neoplasms. J Clin Endocrinol Metab 1994;78:483-91. [PubMed]
- Liu J, Kahri AI, Heikkila P, et al. Ribonucleic acid expression of the clustered imprinted genes, p57KIP2, insulin-like growth factor II, and H19, in adrenal tumors and cultured adrenal cells. J Clin Endocrinol Metab 1997;82:1766-71. [PubMed]
- Liu J, Kahri AI, Heikkila P, et al. H19 and insulin-like growth factor-II gene expression in adrenal tumors and cultured adrenal cells. J Clin Endocrinol Metab 1995;80:492-6. [PubMed]
- Dall'Igna P, Virgone C, De Salvo GL, et al. Adrenocortical tumors in Italian children: analysis of clinical characteristics and P53 status. Data from the national registries. J Pediatr Surg 2014;49:1367-71. [Crossref] [PubMed]
- Figueiredo BC, Stratakis CA, Sandrini R, et al. Comparative genomic hybridization analysis of adrenocortical tumors of childhood. J Clin Endocrinol Metab 1999;84:1116-21. [PubMed]
- Grimberg A, Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol 2000;183:1-9. [Crossref] [PubMed]
- l'Allemand D, Penhoat A, Blum W, et al. Is there a local IGF-system in human adrenocortical cells? Mol Cell Endocrinol 1998;140:169-73. [Crossref] [PubMed]
- Weber MM, Auernhammer CJ, Kiess W, et al. Insulin-like growth factor receptors in normal and tumorous adult human adrenocortical glands. Eur J Endocrinol 1997;136:296-303. [Crossref] [PubMed]
- Gicquel C, Bertagna X, Gaston V, et al. Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Res 2001;61:6762-7. [PubMed]
- Gicquel C, Raffin-Sanson ML, Gaston V, et al. Structural and functional abnormalities at 11p15 are associated with the malignant phenotype in sporadic adrenocortical tumors: study on a series of 82 tumors. J Clin Endocrinol Metab 1997;82:2559-65. [PubMed]
- Ilvesmaki V, Blum WF, Voutilainen R. Insulin-like growth factor binding proteins in the human adrenal gland. Mol Cell Endocrinol 1993;97:71-9. [Crossref] [PubMed]
- Stratakis CA, Boikos SA. Genetics of adrenal tumors associated with Cushing's syndrome: a new classification for bilateral adrenocortical hyperplasias. Nat Clin Pract Endocrinol Metab 2007;3:748-57. [Crossref] [PubMed]
- Libe R, Bertherat J. Molecular genetics of adrenocortical tumours, from familial to sporadic diseases. Eur J Endocrinol 2005;153:477-87. [Crossref] [PubMed]
- Sparago A, Cerrato F, Vernucci M, et al. Microdeletions in the human H19 DMR result in loss of IGF2 imprinting and Beckwith-Wiedemann syndrome. Nat Genet 2004;36:958-60. [Crossref] [PubMed]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 2009;9:550-62. [Crossref] [PubMed]
- de Joussineau C, Sahut-Barnola I, Levy I, et al. The cAMP pathway and the control of adrenocortical development and growth. Mol Cell Endocrinol 2012;351:28-36. [Crossref] [PubMed]
- Bertherat J, Groussin L, Sandrini F, et al. Molecular and functional analysis of PRKAR1A and its locus (17q22-24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res 2003;63:5308-19. [PubMed]
- Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit (PRKAR1A) in patients with the "complex of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas" (Carney complex). Ann N Y Acad Sci 2002;968:3-21. [Crossref] [PubMed]
- Shi Z, Henwood MJ, Bannerman P, et al. Primary pigmented nodular adrenocortical disease reveals insulin-like growth factor binding protein-2 regulation by protein kinase A. Growth Horm IGF Res 2007;17:113-21. [Crossref] [PubMed]
- Shono T, Sakai H, Takehara K, et al. Analysis of numerical chromosomal aberrations in adrenal cortical neoplasms by fluorescence in situ hybridization. J Urol 2002;168:1370-3. [Crossref] [PubMed]
- Volkl TM, Rauh M, Schofl C, et al. IGF-I-IGFBP-3-acid-labile subunit (ALS) complex in children and adolescents with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency (CAH). Growth Horm IGF Res 2011;21:191-8. [Crossref] [PubMed]
- Belgorosky A, Baquedano MS, Guercio G, et al. Expression of the IGF and the aromatase/estrogen receptor systems in human adrenal tissues from early infancy to late puberty: implications for the development of adrenarche. Rev Endocr Metab Disord 2009;10:51-61. [Crossref] [PubMed]
- Baquedano E, Garcia-Caceres C, Diz-Chaves Y, et al. Prenatal stress induces long-term effects in cell turnover in the hippocampus-hypothalamus-pituitary axis in adult male rats. PLoS One 2011;6. [Crossref] [PubMed]
- Scaroni C, Selice R, Benedini S, et al. Adrenal morpho-functional alterations in patients with acromegaly. J Endocrinol Invest 2008;31:602-6. [Crossref] [PubMed]
- Coulter DL. Prevention as a form of support: implications for the new definition. Ment Retard 1996;34:108-16. [PubMed]
- Zhang J, Wang C, Gao J, et al. Adrenal cortical neoplasms: a study of clinicopathological features related to epidermal growth factor receptor gene status. Diagn Pathol 2014;9:19. [Crossref] [PubMed]
- Adam P, Hahner S, Hartmann M, et al. Epidermal growth factor receptor in adrenocortical tumors: analysis of gene sequence, protein expression and correlation with clinical outcome. Mod Pathol 2010;23:1596-604. [Crossref] [PubMed]
- Gicquel C, Le Bouc Y. Molecular markers for malignancy in adrenocortical tumors. Horm Res 1997;47:269-72. [Crossref] [PubMed]
- Guillaud-Bataille M, Valent A, Soularue P, et al. Detecting single DNA copy number variations in complex genomes using one nanogram of starting DNA and BAC-array CGH. Nucleic Acids Res 2004;32. [Crossref] [PubMed]
- de Reynies A, Assie G, Rickman DS, et al. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol 2009;27:1108-15. [Crossref] [PubMed]
- Logie A, Boulle N, Gaston V, et al. Autocrine role of IGF-II in proliferation of human adrenocortical carcinoma NCI H295R cell line. J Mol Endocrinol 1999;23:23-32. [Crossref] [PubMed]
- Drelon C, Berthon A, Val P. Adrenocortical cancer and IGF2: is the game over or our experimental models limited? J Clin Endocrinol Metab 2013;98:505-7. [Crossref] [PubMed]
- Heaton JH, Wood MA, Kim AC, et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and beta-catenin. Am J Pathol 2012;181:1017-33. [Crossref] [PubMed]
- Guo L, Zhao Y, Yang S, et al. An integrated evolutionary analysis of miRNA-lncRNA in mammals. Mol Biol Rep 2014;41:201-7. [Crossref] [PubMed]
- Gao ZH, Suppola S, Liu J, et al. Association of H19 promoter methylation with the expression of H19 and IGF-II genes in adrenocortical tumors. J Clin Endocrinol Metab 2002;87:1170-6. [Crossref] [PubMed]
- Lefloch R, Pouyssegur J, Lenormand P. Total ERK1/2 activity regulates cell proliferation. Cell Cycle 2009;8:705-11. [Crossref] [PubMed]
- De Martino MC, van Koetsveld PM, Feelders RA, et al. The role of mTOR inhibitors in the inhibition of growth and cortisol secretion in human adrenocortical carcinoma cells. Endocr Relat Cancer 2012;19:351-64. [Crossref] [PubMed]
- Cantini G, Lombardi A, Piscitelli E, et al. Rosiglitazone inhibits adrenocortical cancer cell proliferation by interfering with the IGF-IR intracellular signaling. PPAR Res 2008;2008. [Crossref] [PubMed]
- Lin SR, Tsai JH, Yang YC, et al. Mutations of K-ras oncogene in human adrenal tumours in Taiwan. Br J Cancer 1998;77:1060-5. [Crossref] [PubMed]
- Bussey KJ, Demeure MJ. Genomic and expression profiling of adrenocortical carcinoma: application to diagnosis, prognosis and treatment. Future Oncol 2009;5:641-55. [Crossref] [PubMed]
- Kotoula V, Sozopoulos E, Litsiou H, et al. Mutational analysis of the BRAF, RAS and EGFR genes in human adrenocortical carcinomas. Endocr Relat Cancer 2009;16:565-72. [Crossref] [PubMed]
- Tong AL, Wang F, Cui YY, et al. Interaction between Angiotensin II and Insulin/IGF-1 Exerted a Synergistic Stimulatory Effect on ERK1/2 Activation in Adrenocortical Carcinoma H295R Cells. Int J Endocrinol 2016;2016. [Crossref] [PubMed]
- Sirianni R, Zolea F, Chimento A, et al. Targeting estrogen receptor-alpha reduces adrenocortical cancer (ACC) cell growth in vitro and in vivo: potential therapeutic role of selective estrogen receptor modulators (SERMs) for ACC treatment. J Clin Endocrinol Metab 2012;97:E2238-50. [Crossref] [PubMed]
- De Luca A, Avena P, Sirianni R, et al. Role of Scaffold Protein Proline-, Glutamic Acid-, and Leucine-Rich Protein 1 (PELP1) in the Modulation of Adrenocortical Cancer Cell Growth. Cells 2017;6. [Crossref] [PubMed]
- Watanabe M, Noda M, Nakajin S. Effect of epidermal growth factor and prostaglandin on the expression of aromatase (CYP19) in human adrenocortical carcinoma cell line NCI-H295R cells. J Endocrinol 2006;188:59-68. [Crossref] [PubMed]
- Feltus FA, Kovacs WJ, Nicholson W, et al. Epidermal growth factor increases cortisol production and type II 3 beta-hydroxysteroid dehydrogenase/Delta(5)-Delta(4)-isomerase expression in human adrenocortical carcinoma cells: evidence for a Stat5-dependent mechanism. Endocrinology 2003;144:1847-53. [Crossref] [PubMed]
- Doi J, Takemori H, Ohta M, et al. Differential regulation of 3beta-hydroxysteroid dehydrogenase type II and 17alpha-hydroxylase/lyase P450 in human adrenocortical carcinoma cells by epidermal growth factor and basic fibroblast growth factor. J Endocrinol 2001;168:87-94. [Crossref] [PubMed]
- Patterson EE, Holloway AK, Weng J, et al. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer 2011;117:1630-9. [Crossref] [PubMed]
- Soon PS, Tacon LJ, Gill AJ, et al. miR-195 and miR-483-5p Identified as Predictors of Poor Prognosis in Adrenocortical Cancer. Clin Cancer Res 2009;15:7684-92. [Crossref] [PubMed]
- Doghman M, El Wakil A, Cardinaud B, et al. Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. Cancer Res 2010;70:4666-75. [Crossref] [PubMed]
- Nagane M, Lin H, Cavenee WK, et al. Aberrant receptor signaling in human malignant gliomas: mechanisms and therapeutic implications. Cancer Lett 2001;162 Suppl:S17-S21. [Crossref] [PubMed]
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008;358:1160-74. [Crossref] [PubMed]
- Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 2007;21:2683-710. [Crossref] [PubMed]
- Purba ER, Saita EI, Maruyama IN. Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: The "Rotation Model". Cells 2017;6. [Crossref] [PubMed]
- Xu L, Qi Y, Xu Y, et al. Co-inhibition of EGFR and IGF1R synergistically impacts therapeutically on adrenocortical carcinoma. Oncotarget 2016;7:36235-46. [PubMed]
- Wee P, Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel) 2017;9. [PubMed]
- Poli G, Cantini G, Armignacco R, et al. Metformin as a new anti-cancer drug in adrenocortical carcinoma. Oncotarget 2016;7:49636-48. [Crossref] [PubMed]
- Almeida MQ, Fragoso MC, Lotfi CF, et al. Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. J Clin Endocrinol Metab 2008;93:3524-31. [Crossref] [PubMed]
- Haluska P, Worden F, Olmos D, et al. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol 2010;65:765-73. [Crossref] [PubMed]
- Jones RL, Kim ES, Nava-Parada P, et al. Phase I study of intermittent oral dosing of the insulin-like growth factor-1 and insulin receptors inhibitor OSI-906 in patients with advanced solid tumors. Clin Cancer Res 2015;21:693-700. [Crossref] [PubMed]
- Carden CP, Kim ES, Jones RL, et al. Phase I study of intermittent dosing of OSI-906, a dual tyrosine kinase inhibitor of insulin-like growth factor-1 receptor (IGF-1R) and insulin receptor (IR) in patients with advanced solid tumors. J Clin Oncol 2010;28:abstr 2530.
- Fassnacht M, Berruti A, Baudin E, et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol 2015;16:426-35. [Crossref] [PubMed]
- Hantel C, Lewrick F, Reincke M, et al. Liposomal doxorubicin-based treatment in a preclinical model of adrenocortical carcinoma. J Endocrinol 2012;213:155-61. [Crossref] [PubMed]
- Naing A, Kurzrock R, Burger A, et al. Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. Clin Cancer Res 2011;17:6052-60. [Crossref] [PubMed]
- Lerario AM, Worden FP, Ramm CA, et al. The combination of insulin-like growth factor receptor 1 (IGF1R) antibody cixutumumab and mitotane as a first-line therapy for patients with recurrent/metastatic adrenocortical carcinoma: a multi-institutional NCI-sponsored trial. Horm Cancer 2014;5:232-9. [Crossref] [PubMed]
- Faria AM, Almeida MQ. Differences in the molecular mechanisms of adrenocortical tumorigenesis between children and adults. Mol Cell Endocrinol 2012;351:52-7. [Crossref] [PubMed]
- Barlaskar FM, Spalding AC, Heaton JH, et al. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab 2009;94:204-12. [Crossref] [PubMed]
- Gagliano T, Gentilin E, Tagliati F, et al. Inhibition of epithelial growth factor receptor can play an important role in reducing cell growth and survival in adrenocortical tumors. Biochem Pharmacol 2015;98:639-48. [Crossref] [PubMed]
- Quinkler M, Hahner S, Wortmann S, et al. Treatment of advanced adrenocortical carcinoma with erlotinib plus gemcitabine. J Clin Endocrinol Metab 2008;93:2057-62. [Crossref] [PubMed]


