Determinants of systemic venous return and the impact of positive pressure ventilation
What is venous return and what are its determinants?
When Patterson and Starling described what became later known as the Starling mechanism of the heart, their first conclusion reads: “The output of the heart is equal to and determined by the amount of blood flowing into the heart, and may be increased or diminished within very wide limits according to the inflow” (1).
This simple yet very logical statement has several consequences:
- Cardiac output is governed and thus primarily limited by venous inflow (1);
- The venous circulation, as main reservoir of blood, gains an active role in regulation of cardiac output, rather than being a passive return conduit (2,3);
- The heart acts permissively to promote this venous return (2);
- The Starling mechanism, i.e., the dependency of stroke volume on ventricular pressure, is not the primary determinant of cardiac output, but an adaptive mechanism to accommodate for short-term changes in venous inflow (2,4).
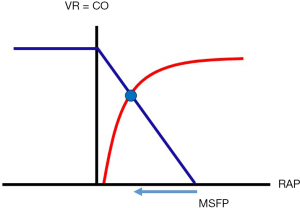
It took more than half a century after the description of the Starling mechanism, until Arthur Guyton experimentally characterized venous return and its respective components (5-7). In order to understand the conceptual framework for venous return, it is important to divide the circulation into two subsystems, the heart as the pump and the vasculature as the circuit. The invention of extracorporeal pumps allowed Guyton to control the heart function via the speed of a mechanical pump and separate pump effects from the vascular tree as the circuit. By systematic and stepwise elevation of right atrial pressure (RAP), he showed an inverse relationship of venous return to RAP (Figure 1) (6-8). He achieved maximum venous return with zero RAP. Further increases in flow via increases in pump function were limited by collapse of the intrathoracic vessels (9). When he increased RAP above zero (i.e., above atmospheric pressure), pump flow and therefore venous return would decline until flow ceased completely. He termed the pressure at zero flow mean circulatory filling pressure (MCFP) (6,7). By influencing MCFP via volume expansion or epinephrine, he could increase venous return without changes in pump function (7,8,10). From this, Guyton reasoned that in the steady state circulation, venous return (and therefore cardiac output in conclusion) was driven by the venous return driving pressure (VRdP = MCFP minus RAP) divided by the resistance to venous return (RVR):
CO = VR = (MCFP – RAP)/RVR [1]
This simple ohmic representation of the circulation allows that the heart acts permissively by pumping forward what it was offered by the venous system. As it can only promote what flows into the heart, cardiac output is completely dependent on venous return in physiological (or non-heart failure) conditions. It could therefore not be possible to increase cardiac output (= stroke volume times heart rate) without simultaneous increases in venous return, for example by increasing heart rate at stable venous return. This was proven several years after the initial derivation of the venous return concept (11,12). Cardiac output can only be elevated when the VRdP is increased, by either increases in upstream or decreases in downstream pressure (which would be the result of increased cardiac function), or by decreasing RVR (3).
Despite relevant criticism on Guyton’s concept (13-15), its functional consequences have been reproduced in various animal (16-20) and clinical experiments (21-26), with and without mechanical circulatory assist. This concept provides a useful framework that integrates blood volume, central venous pressure or RAP and cardiac output and delineates circulatory factors from cardiac limits in states of shock (27-29).
We will describe the components of venous return, i.e., mean circulatory and systemic filling pressure (MCFP and MSFP), RAP and VRdP, the RVR and link these components to the blood volume within the circulation. Special emphasis will lay on the influences of changing intrathoracic pressures due to mechanical ventilation and the applicability of the concept in dynamic situations, as these form the basis around functional hemodynamic monitoring and heart lung interactions (30-32). The controversies around the concept and varying interpretations will be discussed at each individual component (33,34).
What are the components of venous return and how do they relate to blood volume?
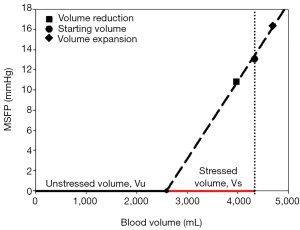
The blood volume in a steady state is relatively constant and due to the much larger venous than arterial compliance, up to 70% of the volume resides in the venous system (35). Blood flow will only redistribute a small amount of around 10% from the venous to the arterial system. Only part of this volume creates tension on the vascular walls, evoking the elastic recoil pressure. This “distending volume” is called stressed volume (35-37). Stressed volume is surprisingly small, around 25% of the total blood volume. The larger part of total blood volume just fills the vascular structures without creating any tension. This unstressed volume does not contribute to flow, but serves as a volume reserve for the body. Unstressed volume may be recruited into stressed volume via changes in the capacitance of the vessel beds, a protective mechanism for cardiac output in hypovolemia.
Blood pressure at zero blood flow was examined long before Guyton. Weber described “statischer Füllungsdruck” (static filling pressure) in 1851 (38). Guyton’s observation of ceasing blood flow when RAP exceeded a certain value led to the conclusion that, as described in formula (1), there is an upstream pressure (MCFP) that drives venous return against RAP (or central venous pressure) as downstream pressure and against RVR. MCFP is the pressure in the whole circulation at zero blood flow after pressure equilibration in the entire vascular bed, including the heart chambers and the lung (39) and represents the elastic recoil of the whole vasculature as a function of total blood volume and the overall vascular compliance (3,38).
Mean systemic filling pressure is the elastic recoil pressure of the systemic vasculature, excluding the volume and compliances of the heart and lung (40,41). This parameter focuses on the return function of the systemic circuit, which is most relevant for the description of altered vascular states and the clinical applications of the concept (19,42). As MSFP excludes the central part of the circulation, it is not only dependent on the blood volume (described above), but also on volume shifts from the central (i.e., heart chambers and pulmonary vascular bed) to peripheral beds prior to the stop flow (20,35,41,43,44).
MSFP is a function of systemic vascular compliance and blood volume (40,41), not vice versa, i.e., the volume and the elastic properties of the vasculature determine the pressure (35). Stressed and unstressed blood volume can be estimated from a step change in MSFP caused by volume infusion or bleeding and measurements of blood volume (Figure 2) (24,41,45).
There is considerable confusion about MSFP and MCFP in the literature (38) and the exclusion of the pulmonary vasculature is a source of criticism for the concept of venous return (34). A distinction of MCFP and MSFP may clinically not be important because they are very similar in value and difficult to estimate or differentiate exactly. Still, when discussing the effects of intrathoracic pressures, lung inflation may shift part of the pulmonary and cardiac blood volume towards the systemic circulation, thereby increasing MSFP while keeping MCFP constant (43,44). With regards to the systemic circulation and its role for various disease states in critical care, we rely on the description of MSFP and formula 1 can be rewritten
CO=VR = (MSFP – RAP)/RVR [2]
The upstream pressure MSFP may be interpreted in two ways. The first interpretation sees MSFP as the pivot pressure of the systemic circulation (3,39), the second as the averaged systemic pressure weighted by vessel compliances, thereby representing the systemic stressed blood volume (37,41,46). In any case, the pressure gradient necessary for blood flow is created by the heart lowering the RAP (20).
If a circulation is restarted from this equilibrated standstill pressure MSFP, volume is redistributed by the heart according to the compliances of the various vascular beds around this pivot pressure (Figure 3). At the pivot, the pressure is primarily independent of the heart (3), stressing the “vascular nature” of this pressure. It is easily illustrated that the pumping of the heart shifts volume according to the compliances of the vascular segments around this pivot pressure. The pivot also offers a run-off pressure behind the arteriolar system and capillary vessels, which explains how blood flow can continue behind arteriolar beds that show critical closing pressures and vascular waterfalls (21,47). Critics argue that a pressure defined for circulatory standstill cannot be present within an ongoing circulation and its exact location in the vasculature would be unknown. Further, such pressures could by no means drive flow at steady state (13,14,48,49), because emptying would decrease the pressure without constant refill. These critics ignore that the emptying mechanics including a highly elastic venous reservoir, are central for the achievable flow (44,50). In addition, since stressed volume is present during ongoing circulation, so must be its related pressure (40). The large compliance of the veins will keep MSFP constant, because it will damp the effect of a stroke volume on the pressure (35,41). So, the function of the heart can be seen as continuous restoration of stressed volume in the circuit (20,40,41). There is experimental evidence (51) that the splanchnic region may operate on pressures close to MSFP. The splanchnic vascular beds have the theoretical prerequisites of low resistances and high capacitance (52).
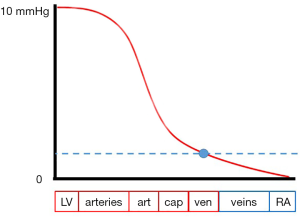
We favour the interpretation of MSFP as averaged pressure in the systemic vasculature, weighted by the compliances of the individual segments (20,40,41). This interpretation is useful when dealing with systemic stressed blood volume and its influence on cardiac output (37) and allows for the explanation of volume shifts from central to peripheral circulations (43,44), which will have an important role in the second part of this article. Accordingly, the RVR should also be interpreted as the resistance encountered by the average systemic circulatory element (46), excluding lung and heart.
Repessé et al. measured MSFP in critically ill dead patients and found a mean value of 12.8±5.6 mmHg (53), one minute after the heart stopped beating, possibly influenced by ongoing reflexes (54) and exchange of volume from lungs and heart. Estimates with extrapolation methods for critically ill patients with beating hearts range from 18 mmHg up to 33 mmHg (23). These values are much higher than from animal experiments with controlled conditions, were values below 10 mmHg are found (41,55). MSFP seems to be constant between species (35).
The downstream pressure in Guyton’s concept is RAP. At a constant MSFP, the higher the RAP, the lower the VR and cardiac output and vice versa. RAP at the intersection of the Starling curve with the venous return curve represents the equilibrium point at which a given cardiac function and vascular circuit can work (Figure 1) (6,20). Remember that Starling’s experiment was done in open atmosphere, not within a closed thorax, i.e., transmural RAPs were not influenced by intrathoracic pressures swings. If RAP, measured towards atmosphere, falls because of lower intrathoracic pressure, like during spontaneous inspiration, both venous return and transmural RAP will increase (56).
The downstream role of RAP is central for heart lung interactions (18,19). Still there are alternative interpretations of its role, leading to heavy critique on the VR concept (15,34). Guyton used a movable Starling resistor to manipulate RAP while keeping the volume in the circulation constant and his preparation only bypassed the right heart, feeding the blood back into the pulmonary artery (8). Critics say that RAP could not be the independent backpressure to VR because it was altered via the use of a Starling resistor and that the heart function was not completely controlled for, because the left ventricle was still functioning (15). Levy performed a right heart bypass experiment and altered RAP with changing pump speed. Guyton had altered RAP via the height of a collapsible tube (Starling resistor). Levy interpreted RAP solely as consequence of altered flow (33). This opposite interpretation of cause and effect from Guyton could not be resolved in an ongoing theoretical debate (13-15,40,48-50,52,57-64). We have recently performed a porcine experiment without starling resistors and with a complete heart bypass and ligation of the pulmonary artery to get full control of the pump and volume shifts due to lung inflation. We altered RAP with the pump to define the relationship of RAP and pump function and then altered RAP at constant pump function by changing airway pressure. We could obviate all elements of former criticisms and verify the role of RAP as backpressure to venous return (20). This confirmed previous similar experiments with beating hearts (18,19).
Behind this theoretical cause-and-effect discussion, still unresolved for some (65), the clinician must realize that RAP itself is highly influenced by intrathoracic pressure conditions, lung inflation and lung compliance, pericardial constraint and cardiac function (66,67). Behind this complexity, small changes in RAP may contribute heavily to changes in VRdP. The VRdP is small, just some mmHg. The changes in RAP caused by cardiac pump function and changes in intrathoracic pressure are large in comparison the oscillations of MSFP. Changes in RAP created by stroke volume and respiratory cycle will always predominate changes in MSFP, as the large vessel compliance limits the pressure effect of a single stroke volume on MSFP (37).
Changing intrathoracic pressures have large effects on RAP. In spontaneous breathing, pleural pressure is constantly negative and the transmural RAP remains positive even if RAP measured towards atmosphere is zero. Zero RAP leads to maximum venous return. With mechanical ventilation, pleural pressure rises and may even become positive. RAP (towards atmosphere) rises, thereby reducing VRdP while transmural RAP (inside minus outside pressure) falls (56). Rises in RAP may also be caused by increased right ventricular afterload during mechanical ventilation (67).
How can the determinants of VR be assessed at the bedside?
Cardiac output and central venous or RAP are readily available at the bedside and reliable, when proper zeroing and levelling is taken care of (68). The true effectors of venous return and cardiac output, i.e., stressed vascular volume, vascular compliance and resistance cannot be assessed during ongoing circulation (65).
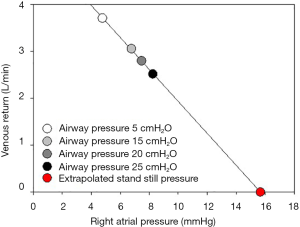
In the physiology lab, a standstill pressure can be measured by various means [ventricular fibrillation (69), stop of extracorporeal circulation (19,20), right atrial balloon obstruction (41,45) or acetylcholine (51)]. Each procedure results in slightly different values depending on volume shifts from the lung (39,44). If volume shifts are possible, like in ventricular fibrillation, MCFP is measured. With obstruction of the right atrium, MSFP is obtained. Such measurements are not feasible at the bedside. Clinical assessment relies on surrogate pressures similar to MSFP and extrapolations. Besides mathematical modelling of a MSFP analogue (66,70,71), and exclusion of the extremity vasculature with a high pressure cuff (26,72), clinically applicable methods rely on heart lung interactions. Increases in intrathoracic pressure and thereby RAP will lower venous return and cardiac output by reducing VRdP. When RAP and cardiac output pairs are measured at different airway pressures, a standstill pressure or MSFP can be extrapolated by linear regression (Figure 4). These measurements where introduced in animal models by Versprille and Jansen for stepwise elevations in plateau pressure (18) or slow single breath inflation by Pinsky (19), verifying the conceptual framework for heart lung interactions. Recently, these manoeuvers were brought to the bedside by Maas and colleagues and others in a series of experiments, revealing rather high MSFP estimates (21-26,73,74). These methods rely on stable MSFP as upstream pressure for venous return and changing RAP. We have recently observed that such manoeuvers may cause a rightward shift of the VR curve and lead to an overestimation of MSFP measured with a balloon occlusion of the right atrium (41). Possible mechanisms are discussed below.
How do airway and intrathoracic pressures influence venous return and particularly MSFP?
Tidal changes in intrathoracic pressures due to mechanical ventilation constitute dynamic transients. The VR concept was formulated for steady state conditions. RVR and MSFP are usually considered to be stable and constant (19) and the main effect of intrathoracic pressures therefore lays on RAP as backpressure to venous return, as described above (19,20). MSFP results from stressed vascular volume and systemic vascular compliance or capacitance. If MSFP changes, one or all of its determinants must change. Intrathoracic pressures may therefore affect MSFP:
- Central (i.e., lung and heart) compartments or the splanchnic region may exchange stressed volume with the systemic circulation;
- Intermittent vessel closure due to high surrounding pressures may disrupt the classical upstream downstream pressure gradient;
- The effects of static airway pressure (positive end-expiratory airway pressure) or tidal ventilation (plateau pressure) on venous return may differ.
The most abrupt influence of intrathoracic pressures on venous return are vascular waterfalls (9). When transmural vessel pressure (inside minus outside pressure) approaches zero due to high external pleural pressure (41), the relationship of RAP to MSFP is disrupted. Guyton recognized such waterfalls and closing conditions as the main limit to increases in flow. Closing conditions were observed in animal models (75) and by echocardiography in the caval veins in critically ill patients and related to their volume status (76,77). The great veins tend to collapse more easily that the right atrium, so that the zone of collapse is considered to be at the cavo-atrial border (78). Compression of the great veins may influence the resistance to venous return.
The clinical observation that high intrathoracic pressures lower cardiac output is believed to be mainly an effect of elevated RAP and therefore reduced VRdP. Experimental results are controversial. For static pressure changes (increases in positive end-expiratory airway pressure), VRdP is maintained by increased MSFP. Fessler et al. found stable VRdP between zero and 15 cmH2O PEEP, with similar increases in RAP and MSFP despite falling venous return. They conclude that PEEP increases resistance to venous return via reflexes and mechanical factors independent of abdominal pressure (79) and later verified their results in a heart bypass model (80). Nanas and Magder confirm the stable VRdP between 10 and 20 cmH2O of PEEP with falling cardiac output and therefore increasing resistance to venous return (45). Jellinek et al. found similar results of stable VRdP and increasing RVR in patients undergoing testing of implanted cardioverter-defibrillators at sustained inflation (81). Chihara et al. describes unchanged RVR with decreasing driving pressure in a rat model (82). We investigated lower levels of PEEP (5 to 10 cmH2O) with stable airway plateau pressures and found no effects on venous return, MSFP or RVR. Our model reflected current ventilation practice with low tidal volumes and limited airway driving pressures (41). These smaller pressures and volumes may explain the different results to the older studies, together with real time stroke volume measurement compared to thermodilution. The mechanisms by which PEEP increases MSFP are not clear. Nanas found a decrease in vascular capacitance with increasing stressed volume (45). Pressurisation of the abdominal compartment was proposed (83,84), but not confirmed (80). Based on our findings of a volume dependent leftward shift of the venous return curve with inspiratory hold maneuvers, we have proposed a hepatosplanchnic waterfall, which could recruit volume (41,85,86).
During tidal ventilation, MSFP is theoretically held constant by the mechanical properties of the vasculature. The time constant for emptying the venous system is much longer than a normal respiratory cycle (19), limiting the possibility for MSFP to fall in expiration. The changes in stroke volumes during tidal ventilation are small and dampened by the large vessel compliance, so that MSFP may not rise by small volume changes caused by decreasing stroke volumes when RAP is increased (18,19).
Repessé et al. recently reported that tidal inflation increased MSFP by around 2 mmHg with an airway driving pressure of 14 cmH2O in critically ill patients immediately after cardiac arrest (87). Due to the short duration of respiratory cycle, reflex adaption is an unlikely mechanism, since reflex increases in MSFP become apparent first after roughly 10 seconds (20,41). The observed increase in inspiratory MSFP matches the estimated volume shift from the lungs into the systemic circulation (87). Such volume shifts from the lung or central compartment to the periphery are well known (88,89), but considered small (41,90). An experimental proof of pulmonary contribution to changing MSFP is lacking. Nevertheless, changing MSFP in dynamic short-term situations implies acute shifts in stressed volume. These can occur between the central and peripheral compartment (43) or within the systemic circulation (41). We have recently shown in an animal model with complete heart-lung bypass and excluded pulmonary vasculature that VRdP may dynamically change during tidal ventilation. The effects of airway pressures on VR seem to be dominated by their effect on the downstream pressure, i.e., on RAP. Most importantly, the VR concept, formulated for steady state conditions, is valid and useful in dynamic situations (20), even though the exact values of the upstream pressure MSFP may be undeterminable.
What are the clinical consequences?
The concept of venous return provides a useful framework that integrates blood volume, vascular and cardiac function. Starling’s observation that the heart pumps what it gets back from the body stressed the interplay between the heart and the vasculature. In cardiology and critical care, the Starling mechanism has dominated the thinking and interpretation of shock states. But it is the VR concept that enables a focus on prevalent clinical problems in the ICU like right heart failure or vasoplegia. It forms the basis for heart lung interactions and functional hemodynamic monitoring.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Patterson SW, Starling EH. On the mechanical factors which determine the output of the ventricles. J Physiol 1914;48:357-79. [Crossref] [PubMed]
- Guyton AC. Regulation of cardiac output. Anesthesiology 1968;29:314-26. [Crossref] [PubMed]
- Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology 2008;108:735-48. [Crossref] [PubMed]
- Berlin DA, Bakker J. Starling curves and central venous pressure. Crit Care 2015;19:55. [Crossref] [PubMed]
- Andrew P. CrossTalk proposal: Guyton's venous return curves should be taught. J Physiol 2013;591:5791-3. [Crossref] [PubMed]
- Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev 1955;35:123-9. [PubMed]
- Guyton AC, Lindsey AW, Kaufmann BN. Effect of mean circulatory filling pressure and other peripheral circulatory factors on cardiac output. Am J Physiol 1955;180:463-8. [PubMed]
- Guyton AC, Lindsey AW, Abernathy B, et al. Venous return at various right atrial pressures and the normal venous return curve. Am J Physiol 1957;189:609-15. [Crossref] [PubMed]
- Permutt S, Riley RL. Hemodynamics of collapsible vessels with tone: the vascular waterfall. J Appl Physiol 1963;18:924-32. [Crossref] [PubMed]
- Guyton AC, Lindsey AW, Abernathy B, et al. Mechanism of the increased venous return and cardiac output caused by epinephrine. Am J Physiol 1958;192:126-30. [Crossref] [PubMed]
- Cowley AW Jr, Guyton AC. Heart rate as a determinant of cardiac output in dogs with arteriovenous fistula. Am J Cardiol 1971;28:321-5. [Crossref] [PubMed]
- Ross J Jr, Linhart JW, Brauwald E. Effects of changing heart rate in man by electrical stimulation of the right atrium. studies at rest, during exercise, and with isoproterenol. Circulation 1965;32:549-58. [Crossref] [PubMed]
- Brengelmann GL. The classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is/is not correct. J Appl Physiol (1985) 2006;101:1532. [Crossref] [PubMed]
- Brengelmann GL. Counterpoint: the classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is not correct. J Appl Physiol (1985) 2006;101:1525-6; discussion 1526-7. [Crossref] [PubMed]
- Brengelmann GL. A critical analysis of the view that right atrial pressure determines venous return. J Appl Physiol (1985) 2003;94:849-59. [Crossref] [PubMed]
- Den Hartog EA, Versprille A, Jansen JR. Systemic filling pressure in intact circulation determined on basis of aortic vs. central venous pressure relationships. Am J Physiol 1994;267:H2255-8. [PubMed]
- Hartog EA, Jansen JR, Moens GH, et al. Systemic filling pressure in the intact circulation determined with a slow inflation procedure. Pflugers Arch 1996;431:863-7. [Crossref] [PubMed]
- Versprille A, Jansen JR. Mean systemic filling pressure as a characteristic pressure for venous return. Pflugers Arch 1985;405:226-33. [Crossref] [PubMed]
- Pinsky MR. Instantaneous venous return curves in an intact canine preparation. J Appl Physiol Respir Environ Exerc Physiol 1984;56:765-71. [PubMed]
- Moller PW, Winkler B, Hurni S, et al. Right atrial pressure and venous return during cardiopulmonary bypass. Am J Physiol Heart Circ Physiol 2017;313:H408-h20. [Crossref] [PubMed]
- Maas JJ, de Wilde RB, Aarts LP, et al. Determination of vascular waterfall phenomenon by bedside measurement of mean systemic filling pressure and critical closing pressure in the intensive care unit. Anesth Analg 2012;114:803-10. [Crossref] [PubMed]
- Maas JJ, Geerts BF, Jansen JR. Evaluation of mean systemic filling pressure from pulse contour cardiac output and central venous pressure. J Clin Monit Comput 2011;25:193-201. [Crossref] [PubMed]
- Maas JJ, Geerts BF, van den Berg PC, et al. Assessment of venous return curve and mean systemic filling pressure in postoperative cardiac surgery patients. Crit Care Med 2009;37:912-8. [Crossref] [PubMed]
- Maas JJ, Pinsky MR, Aarts LP, et al. Bedside assessment of total systemic vascular compliance, stressed volume, and cardiac function curves in intensive care unit patients. Anesth Analg 2012;115:880-7. [Crossref] [PubMed]
- Maas JJ, Pinsky MR, de Wilde RB, et al. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med 2013;41:143-50. [Crossref] [PubMed]
- Maas JJ, Pinsky MR, Geerts BF, et al. Estimation of mean systemic filling pressure in postoperative cardiac surgery patients with three methods. Intensive Care Med 2012;38:1452-60. [Crossref] [PubMed]
- Funk DJ, Jacobsohn E, Kumar A. The role of venous return in critical illness and shock-part I: physiology. Crit Care Med 2013;41:255-62. [Crossref] [PubMed]
- Funk DJ, Jacobsohn E, Kumar A. Role of the venous return in critical illness and shock: part II-shock and mechanical ventilation. Crit Care Med 2013;41:573-9. [Crossref] [PubMed]
- Magder S, Vanelli G. Circuit factors in the high cardiac output of sepsis. J Crit Care 1996;11:155-66. [Crossref] [PubMed]
- Cavallaro F, Sandroni C, Antonelli M. Functional hemodynamic monitoring and dynamic indices of fluid responsiveness. Minerva Anestesiol 2008;74:123-35. [PubMed]
- Pinsky MR, Payen D. Functional hemodynamic monitoring. Crit Care 2005;9:566-72. [Crossref] [PubMed]
- Magder S. Venous Return. In: Scharf S. editor. Respiratory-Circulatory Interactions in Health and Disease. New York: Marcel Dekker, 2001.
- Levy MN. The cardiac and vascular factors that determine systemic blood flow. Circ Res 1979;44:739-47. [Crossref] [PubMed]
- Beard DA, Feigl EO. Understanding Guyton's venous return curves. Am J Physiol Heart Circ Physiol 2011;301:H629-33. [Crossref] [PubMed]
- Magder S. How Does Volume Make the Blood Go Around. In: Vincent JL. editor. Annual Update in Intensive Care and Emergency Medicine 2015. Springer, 2015.
- Magder S, De Varennes B. Clinical death and the measurement of stressed vascular volume. Crit Care Med 1998;26:1061-4. [Crossref] [PubMed]
- Magder S. Volume and its relationship to cardiac output and venous return. Crit Care 2016;20:271. [Crossref] [PubMed]
- Henderson WR, Griesdale DE, Walley KR, et al. Clinical review: Guyton--the role of mean circulatory filling pressure and right atrial pressure in controlling cardiac output. Crit Care 2010;14:243. [Crossref] [PubMed]
- Rothe CF. Mean circulatory filling pressure: its meaning and measurement. J Appl Physiol (1985) 1993;74:499-509. [Crossref] [PubMed]
- Berger D, Moller PW, Takala J. Reply to “Letter to the editor: Why persist in the fallacy that mean systemic pressure drives venous return?”. Am J Physiol Heart Circ Physiol 2016;311:H1336-H7.
- Berger D, Moller PW, Weber A, et al. Effect of PEEP, blood volume, and inspiratory hold maneuvers on venous return. Am J Physiol Heart Circ Physiol 2016;311:H794-806. [Crossref] [PubMed]
- Lee JM, Ogundele O, Pike F, et al. Effect of acute endotoxemia on analog estimates of mean systemic pressure. J Crit Care 2013;28:880.e9-15. [Crossref] [PubMed]
- Mitzner W, Goldberg H, Lichtenstein S. Effect of thoracic blood volume changes on steady state cardiac output. Circ Res 1976;38:255-61. [Crossref] [PubMed]
- Magder S, Veerassamy S, Bates JH. A further analysis of why pulmonary venous pressure rises after the onset of LV dysfunction. J Appl Physiol (1985) 2009;106:81-90. [Crossref] [PubMed]
- Nanas S, Magder S. Adaptations of the peripheral circulation to PEEP. Am Rev Respir Dis 1992;146:688-93. [Crossref] [PubMed]
- Sondergaard S, Parkin G, Aneman A. Central venous pressure: soon an outcome-associated matter. Curr Opin Anaesthesiol 2016;29:179-85. [Crossref] [PubMed]
- Magder S. Starling resistor versus compliance. Which explains the zero-flow pressure of a dynamic arterial pressure-flow relation? Circ Res 1990;67:209-20. [Crossref] [PubMed]
- Brengelmann GL. Steady-state venous return: residue in a recent model analysis of the notion that it is driven by elastic recoil of the venous system. J Appl Physiol (1985) 2009;107:369-author reply 70. [Crossref] [PubMed]
- Brengelmann GL. Letter to the editor: Comments on "Value and determinants of the mean systemic filling pressure in critically ill patients". Am J Physiol Heart Circ Physiol 2015;309:H1370-1.
- Permutt S. The classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is/is not correct. J Appl Physiol (1985) 2006;101:1528. [Crossref] [PubMed]
- Gaddis ML, Rothe CF, Tunin RS, et al. Mean circulatory filling pressure: potential problems with measurement. Am J Physiol 1986;251:H857-62. [PubMed]
- Repesse X, Vieillard-Baron A. Reply to "Letter to the editor: Comments on 'Value and determinants of the mean systemic filling pressure in critically ill patients'". Am J Physiol Heart Circ Physiol 2015;309:H1372-3.
- Repessé X, Charron C, Fink J, et al. Value and determinants of the mean systemic filling pressure in critically ill patients. Am J Physiol Heart Circ Physiol 2015;309:H1003-7. [Crossref] [PubMed]
- Berger D, Moller PW, Bloechlinger S, et al. Methodological issues for the determination of mean systemic filling pressure at the end of life. Critical Care 2017;21:106.
- Guyton AC, Polizo D, Armstrong GG. Mean circulatory filling pressure measured immediately after cessation of heart pumping. Am J Physiol 1954;179:261-7. [Crossref] [PubMed]
- Grubler MR, Wigger O, Berger D, et al. Basic concepts of heart-lung interactions during mechanical ventilation. Swiss Med Wkly 2017;147. [PubMed]
- Brengelmann GL. Why persist in the fallacy that mean systemic pressure drives venous return? Am J Physiol Heart Circ Physiol 2016;311:H1333-5. [Crossref] [PubMed]
- Brengelmann GL. Learning opportunities in the study of Curran-Everett's exploration of a classic paper on venous return. Adv Physiol Educ 2008;32:242-3. [Crossref] [PubMed]
- Magder S. The classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is/is not correct. J Appl Physiol (1985) 2006;101:1533. [Crossref] [PubMed]
- Magder S. Point: the classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is/is not correct. J Appl Physiol (1985) 2006;101:1523-5. [Crossref] [PubMed]
- Baker RD. The classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is/is not correct. J Appl Physiol (1985) 2006;101:1528. [Crossref] [PubMed]
- Mitzner W. The classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is/is not correct. J Appl Physiol (1985) 2006;101:1528. [Crossref] [PubMed]
- Pinsky MR. The classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is/is not correct. J Appl Physiol (1985) 2006;101:1528. [Crossref] [PubMed]
- Tyberg JV. How changes in venous capacitance modulate cardiac output. Pflugers Arch 2002;445:10-7. [Crossref] [PubMed]
- Kamiya A, Hayama Y, Shimizu S, et al. State-space representation of the extended Guyton's model. Am J Physiol Heart Circ Physiol 2017;313:H320-H2. [Crossref] [PubMed]
- Parkin WG, Leaning MS. Therapeutic control of the circulation. J Clin Monit Comput 2008;22:391-400. [Crossref] [PubMed]
- Pinsky MR. The right ventricle: interaction with the pulmonary circulation. Crit Care 2016;20:266. [Crossref] [PubMed]
- Magder S. Central venous pressure: A useful but not so simple measurement. Crit Care Med 2006;34:2224-7. [Crossref] [PubMed]
- Ogilvie RI, Zborowska-Sluis D, Tenaschuk B. Measurement of mean circulatory filling pressure and vascular compliance in domestic pigs. Am J Physiol 1990;258:H1925-32. [PubMed]
- Parkin WG. Volume state control - a new approach. Crit Care Resusc 1999;1:311-21. [PubMed]
- Parkin WG, Wright CA. Three dimensional closed loop control of the human circulation. Int J Clin Monit Comput 1991;8:35-42. [Crossref] [PubMed]
- Aya HD, Rhodes A, Fletcher N, et al. Transient stop-flow arm arterial-venous equilibrium pressure measurement: determination of precision of the technique. J Clin Monit Comput 2016;30:55-61. [Crossref] [PubMed]
- Persichini R, Silva S, Teboul JL, et al. Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit Care Med 2012;40:3146-53. [Crossref] [PubMed]
- Geerts BF, Maas JJ, Aarts LP, et al. Partitioning the resistances along the vascular tree: effects of dobutamine and hypovolemia in piglets with an intact circulation. J Clin Monit Comput 2010;24:377-84. [Crossref] [PubMed]
- Fessler HE, Brower RG, Shapiro EP, et al. Effects of positive end-expiratory pressure and body position on pressure in the thoracic great veins. Am Rev Respir Dis 1993;148:1657-64. [Crossref] [PubMed]
- Vieillard-Baron A, Augarde R, Prin S, et al. Influence of superior vena caval zone condition on cyclic changes in right ventricular outflow during respiratory support. Anesthesiology 2001;95:1083-8. [Crossref] [PubMed]
- Vieillard-Baron A, Chergui K, Rabiller A, et al. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med 2004;30:1734-9. [Crossref] [PubMed]
- Lansdorp B, Hofhuizen C, van Lavieren M, et al. Mechanical Ventilation-Induced Intrathoracic Pressure Distribution and Heart-Lung Interactions. Crit Care Med 2014;42:1983-90. [Crossref] [PubMed]
- Fessler HE, Brower RG, Wise RA, et al. Effects of positive end-expiratory pressure on the gradient for venous return. Am Rev Respir Dis 1991;143:19-24. [Crossref] [PubMed]
- Fessler HE, Brower RG, Wise RA, et al. Effects of positive end-expiratory pressure on the canine venous return curve. Am Rev Respir Dis 1992;146:4-10. [Crossref] [PubMed]
- Jellinek H, Krenn H, Oczenski W, et al. Influence of positive airway pressure on the pressure gradient for venous return in humans. J Appl Physiol (1985) 2000;88:926-32. [Crossref] [PubMed]
- Chihara E, Hashimoto S, Kinoshita T, et al. Elevated mean systemic filling pressure due to intermittent positive-pressure ventilation. Am J Physiol 1992;262:H1116-21. [PubMed]
- Takata M, Beloucif S, Shimada M, et al. Superior and inferior vena caval flows during respiration: pathogenesis of Kussmaul's sign. Am J Physiol 1992;262:H763-70. [PubMed]
- van den Berg PC, Jansen JR, Pinsky MR. Effect of positive pressure on venous return in volume-loaded cardiac surgical patients. J Appl Physiol (1985) 2002;92:1223-31. [Crossref] [PubMed]
- Krogh A. The Regulation of the Supply of Blood to the Right Heart1. Skandinavisches Archiv Für Physiologie 1912;27:227-48. [Crossref]
- Brienza N, Ayuse T, O'Donnell CP, et al. Regional control of venous return: liver blood flow. Am J Respir Crit Care Med 1995;152:511-8. [Crossref] [PubMed]
- Repesse X, Charron C, Geri G, et al. Impact of positive pressure ventilation on mean systemic filling pressure in critically ill patients after death. J Appl Physiol (1985) 2017;122:1373-8. [Crossref] [PubMed]
- Vieillard-Baron A, Chergui K, Augarde R, et al. Cyclic changes in arterial pulse during respiratory support revisited by Doppler echocardiography. Am J Respir Crit Care Med 2003;168:671-6. [Crossref] [PubMed]
- Versprille A, Jansen JR. Tidal variation of pulmonary blood flow and blood volume in piglets during mechanical ventilation during hyper-, normo- and hypovolaemia. Pflugers Arch 1993;424:255-65. [Crossref] [PubMed]
- Brower R, Wise RA, Hassapoyannes C, et al. Effect of lung inflation on lung blood volume and pulmonary venous flow. J Appl Physiol (1985) 1985;58:954-63. [Crossref] [PubMed]


