Interpretation of the transpulmonary pressure in the critically ill patient
Introduction: mechanical ventilation and ventilator-induced lung injury (VILI)
As a part of the supportive therapy of modern intensive care medicine, the majority of critically ill patients undergo invasive mechanical ventilation during their stay in the intensive care unit. However, mechanical ventilation does support the function of the lungs; instead, from a pathophysiological point of view, it is a substitute for the activity of respiratory muscles, which is undertaken to buy time for healing to take place (1).
Of note, soon after its introduction into modern critical care (2), it was discovered how mechanical ventilation itself could lead to a structural damage to the lung (3). Indeed, a completely “safe” form of mechanical ventilation has not been found yet, as the main side-effects associated with this technique are the hemodynamic instability due to the increased intrathoracic pressures, and the direct mechanical trauma to the structure of the lungs. In fact, it has repeatedly been shown how mechanical ventilation itself can lead to worsening injury of previously damaged lungs, or it can damage the lungs even in the absence of a pre-existing lung injury. This injury has collectively been termed VILI (4). Two main mechanisms may injure the lung: firstly, excessively high inspiratory pressures and volumes (respectively identified by lung stress and strain), leading to an excessive distention of the alveolar wall, may cause injurious stretching/overdistention in the lung parenchyma (4,5). On the other side, VILI may occur when the airway pressure (Paw) and expiratory volume are too low, as a consequence of inadequate positive end-expiratory pressure (PEEP) levels, as this may cause cyclic alveolar recruitment/derecruitment with each breath and an excessive tension at margins between aerated/nonaerated lung regions.
In an attempt to reduce the iatrogenic load secondary to the delivery of mechanical ventilation, extensive research has been conducted to identify less injurious ventilator strategies (6). The so-called “protective ventilation” is a paradigm that aims to an individual tailoring of ventilatory support. The current mainstay of such an approach are a low tidal volume ventilation, and the avoidance of elevated Paws. Indeed, even with such an approach, it was shown how a significant proportion of critically ill patients may experience some degree of tidal hyperinflation (7), highlighting our still incomplete understanding of the pathophysiology of VILI.
Indeed, while alveolar pressure is relatively easy to estimate clinically as the Paw during a period of zero flow (either during an expiratory, or an inspiratory hold manoeuvre), it only represents the pressure that is distending the respiratory system. Since the lungs and the chest wall are two elastic structures in series, a fraction of ventilator-delivered pressure is dissipated in inflating the chest wall rather than the lung (Figure 1). Measuring the pressure that distends the lungs only, i.e., the transpulmonary pressure, may then be a better approach to guide ventilator management.
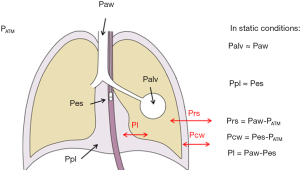
Moreover, as during assisted modes of breathing the patient inspiratory muscles share part of the total work of breathing (WOB) with the mechanical ventilator, so that the pressure which inflates the lungs is the sum of that applied by the ventilator and that applied by the patient, the only way to directly assess the patient contribution to the assisted breath is to measure its muscle pressure, i.e., the negative pleural pressure (Ppl) generated by its inspiratory muscles.
The present review examines the characteristics and limitations of the monitoring of airway and transpulmonary pressure, and it highlights the potential application of transpulmonary pressure assessment during both controlled and spontaneous/assisted mechanical ventilation in critically ill patients.
Limits of Paw monitoring
Ever since the early applications of invasive mechanical ventilation as a form of respiratory support for critically ill patients, pressure-based respiratory mechanics have guided the clinicians when adjusting the ventilator (8). However, measurements based only on Paw may not be easily generalized in a patient population with different pathologic conditions. In fact, the main focus of the physician is the mechanical behavior of the passive lungs, while Paw-based interpretations of respiratory mechanics are often influenced by several pathophysiological alterations: differences in breathing pattern, altered chest wall characteristics (often secondary to fluid overload) (9), alterations in lung volume, increased intra-abdominal pressure (IAP) (such as with capillary leak or fluid overload) (10), the extent of lung edema and collapse, the distribution and asymmetry of lung disease (11), the presence and extent of spontaneous breathing efforts (12). It has been shown how all of these factors do complicate the interpretation of respiratory mechanics, thus preventing the interpretation of Paw to be easily generalizable (13).
Setting the ventilator based on Paw measurements may indeed be adequate for most critically ill patients. However, Paw is an oversimplified surrogate for the pressure in the lungs, as the respiratory system is composed of two elastic structures in series, namely the chest wall and the lungs. Indeed, chest wall alterations are common in critically ill patients, and they may not be easily predicted (10,14-17); as a consequence, the contribution of the chest wall to respiratory system mechanics should not be ignored. For each tidal volume, a stiffer chest wall implies the development of higher Ppl, as a greater part of the driving pressure is spent to move the chest wall. As a consequence, the same Paw may lead to dramatically different transpulmonary Ppl depending on the chest wall properties, as exemplified in Figure 2, upper panel. Indeed, the pressure shown on the ventilator display is in fact the Paw, and in the clinical setting, this is the variable more commonly used to assess lung overdistention. However, correct interpretation of this variable requires knowledge of its determinants.
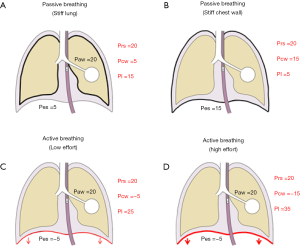
Although the use of low tidal volumes and limited plateau pressures are the current standard of care, this lung protective approach has been shown to provide an inadequate substitute when aiming to assess lung stress and strain, and the suggested limits may not be safe for all patients, depending on their relative characteristics of the chest wall (18,19). To this extent, Chiumello et al. demonstrated how, both in a group of patients with different severity of lung injury and in control medical and surgical patients undergoing mechanical ventilation for conditions different from respiratory failure, the ratio between lung and chest wall elastance (Ecw) may range from as low as 0.2 to as high as 0.8, making the interpretation of Paw potentially misleading when extrapolating this data to estimate the pressure distending the lungs (Pl) (18).
In conclusion, the conventional management of ventilation based on Paw-monitoring limits the chances to tailor the ventilator setting at the individual level. Individualized settings of mechanical ventilation may be the only way to provide effective and safe ventilation in more complex patients; to do so, the understanding of the overall influence of all these factors on respiratory system mechanics is of crucial importance.
In recent years, a renewed interest started to raise around the assessment of transpulmonary pressure (Pl, i.e., the pressure distending the lung), and this variable has increasingly been recommended to guide mechanical ventilation and to tailor it at the individual level. This physiologically sound, yet simple bedside tool may help clinicians to improve lung mechanics and gas exchange while at the same time avoiding lung injury in the more complex critically ill patients (20). In the following paragraphs, we will focus on the physiological rationale, measurement techniques and conditions that may influence esophageal pressure (Pes) monitoring, and on the potential clinical applications of transpulmonary pressure monitoring.
Pes monitoring: perks and pitfalls
As we said earlier, prediction of lung mechanical properties from Paw measurements is often misleading, more so when the disease is unevenly distributed or spontaneous breathing efforts are allowed. Indeed, for a given Paw, the portion of the applied pressure which is in fact applied to inflate only the lungs could vary widely, depending on the mechanical characteristics of the chest wall (16). In this context, assessment of Ppl may be helpful to differentiate between patients who may benefit from a higher Paws because of their increased chest wall elastance (Ecw) from those who, despite relatively low levels of Paw, are still at risk of overdistention.
Ppl can experimentally be measured by inserting a device directly into the pleural space (21). However, this technique is invasive and it has never been used in the clinical practice. Moreover, direct introduction of a probe into the pleural space may potentially alter the mechanical characteristic of that space.
In the clinical ground, the conventional estimate of Ppl requires the measurement of Pes with a balloon-tipped catheter, a technique extensively used ever since the fifties for the physiological investigations of respiratory mechanics. Briefly, Pes is considered to be representative of the average value of Ppl surrounding the lungs, although this assumption is largely based upon studies in healthy subjects in the upright position (22-25). This assumption is based on the anatomical proximity of the lower third of the esophagus to the pleural space and the transmission of Ppl through its wall, as it mainly acts as a passive membrane.
As appealing as this approach seems to be, only in recent years, evidence for its effectiveness is beginning to be found. However, several authors raised concerns about the accuracy of Pes measurements in supine patients with altered lung function (that is, in different conditions from the “classic” respiratory physiology experiments) (26), and the significance of Pes as a proxy for the relevant Ppl.
In fact, several potential confounders may alter the estimation of Ppl from Pes, as summarized in Table 1. The pressure in the esophageal balloon may be influenced by the elastic recoil of the balloon itself, the elastic recoil of the esophagus and esophageal muscle tone, as well as the pressure transmitted from surrounding structures (26). Moreover, the presence of gravitational forces generates a vertical gradient of Ppl in both the upright and supine positions, so that a single value of Ppl does not exist. Significant variability in the relationship between Ppl and Pes is seen with changes from the upright to supine position even in healthy subjects, so that in the latter position higher values of Pes are found at each lung volume (27), likely as a consequence of the cranial displacement of the diaphragm and the weight of the mediastinum. Indeed, in either position, Pes is believed to correspond to the value of Ppl in the middle of the gravitational plane (28,29).

Full table
Following on the observations made by Agostoni et al. (30), tidal changes in Pes closely correlate with changes in the Ppl applied to the surface of the lung, then allowing to estimate transpulmonary pressure as the difference between alveolar pressure and Pes (31). Pes is believed to represent the local pressure along its own gravitational plane; then, absolute values of Ppl in other parts of the chest may theoretically be different even for patients with healthy lungs. For these reasons, concerns have been raised for the ability of Pes to track changes in Ppl in the supine position. Moreover, elevation of IAP and changes of lung volume secondary to position may also influence the value of Pes (32). When parenchymal consolidation is present, in addition to the gravitational gradient of Ppl, local variations may also be present, because of the resistance to parenchymal shape deformation (33); such diseased lungs are often inhomogeneous and less deformable, and increased inter-regional differences in Ppl due to shape change may occur (34,35).
Another limitation in the estimate of Ppl from Pes is the presence of an asymmetrically compromised lung (36). In an experimental study, the author found how the effect of unilateral pleural effusion caused different volume-altering effect in the two lungs; yet, the calculated transpulmonary pressure did not seem to be affected by fluid instillation, so that a single local pressure could not be used to assess the stresses acting in different areas of a heterogeneous thorax.
In addition, in patients with ARDS, the dependent lung regions collapse under the weight of the superimposed tissue, and a vertical gradient of lung inflation is established (37); as a consequence, the actual value of Pes may be different from the Ppl in the most dependent and nondependent lung regions. Seminal experimental studies by Pelosi et al. demonstrated how the actual value of Pes provided an accurate estimate of the Ppl only in the mid-lung zone (38); however, respiratory changes in Pes closely mirrored the changes in Ppl across the different gravitational areas even in diseased lungs (38).
In summary, actual values of Pes provide an accurate surrogate of Ppl only in a localized area of the lung, while at the same time overestimating or underestimating it in other regions. In this way, the use of directly measured Pes to calculate transpulmonary pressure and guide mechanical ventilation may avoid lung collapse or promote recruitment only in the area around the esophageal catheter; however, this setting may actually induce derecruitment or overdistention in other parts of lung. Despite the numerous shortcomings presented, the possibility to estimate Ppl at the bedside is regarded as a necessary step towards an approach to lung protective ventilation more tailored at the individual level. Far from being perfect, estimations of transpulmonary pressure are considered of invaluable help to allow the evaluation of the interactions between the ventilator setting, the extent of the disease and the individual patient characteristics, a further step towards the provision of precision medicine in respiratory critical care.
Transpulmonary pressure: different definitions and assumptions
The actual stress exerted on the lung tissue is represented by its transmural pressure, i.e., the difference between alveolar and Ppl (Figure 1). Given that during static conditions (as during an inspiratory hold) alveolar and airway opening pressure are the same, the transpulmonary pressure (Pl) is generally estimated as the difference between airway and Ppl. Pl is the pressure that drives the movement of air between the environment and the alveoli, and it may be generated by a negative Ppl as during spontaneous breathing (when inspiratory muscles contract, leading to an increased volume of the chest wall and the generation of a negative pressure), positive as during controlled mechanical ventilation (when the ventilator provides positive pressure at the airway opening) or a combination of the two in assisted modes of breathing.
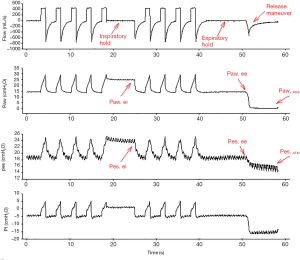
To avoid the pitfalls associated with the well-known limitations of the use of Pes as a surrogate for Ppl, different models have been proposed to estimate Pl; every method for the estimation of Pl is based on assumptions, and there is no clear evidence of the superiority of any of them. Table 2 summarizes the different methods, their assumption and the relevant references; Figure 3 shows representative tracings of airway and Pes used to calculate transpulmonary pressure with the different methods during controlled mechanical ventilation.

Full table
Elastance-derived measurement
In an attempt to remove the confounding influence of the mediastinal weight, assuming that its influence is constant throughout the respiratory cycle, this method, proposed by Gattinoni et al. (16), estimates the end-inspiratory Pl as the product of plateau pressure times the ratio between lung (El) and respiratory system elastance (Ers). Similarly, end-inspiratory Ppl is plateau pressure times the ratio between Ecw and Ers. In other words, this method estimates the portion of Paw that is spent to inflate the lungs only, and the portion that is required to move the chest wall, based on the relative contribution of El and Ecw to Ers.
The assumption underlying this method is that, for mathematical reasons, Pl and Ppl are 0 at atmospheric pressure: if true pressures are higher or lower than 0 when Paw is 0, the calculated pressures will be under or over-estimated, respectively. Secondly, since elastance is calculated using the tidal change in Paw and Ppl, these variations should be linear during tidal inflation. However, elastance may depend on lung volume, and it may lose its linearity at the extremes of the pressure-volume relationship
With this method, given that in a system composed by two elastic structures in series:
Paw = Pl + Ppl and Ers = El + Ecw;
Then
Pl = Paw × El/Ers and Ppl = Paw × Ecw/Ers.
Release-derived measurement
A potential source of bias of the elastance-derived method is that expiratory Pes is measured at PEEP rather than atmospheric pressure. The release-derived method estimates Pl as the change in Paw and Ppl due to both PEEP and tidal ventilation. Pl is then calculated as the difference between Paw and Pes from end-inspiration or end-expiration to atmospheric pressure (18):
Pl = (Paw − Paw at atmospheric pressure) − (Pes − Pes at atmospheric pressure).
Indeed, at end-expiration at atmospheric pressure, Ppl is equated to zero, whatever the absolute value of Pes. This assumption is believed to lead to a lesser bias than assuming Ppl equal to the absolute value of Pes (41). In fact, values as high as 10–15 cmH2O may commonly be found at atmospheric pressure in patients with ARDS, casting doubts as to whether this is compatible at all with an open lung (35,42). In contrast, as seen before, convincing evidence shows how changes in Pes reasonably track the changes in Ppl (21,43,44).
A recent study compared the value of Pl obtained by the elastance-derived method with that measured through a “release” maneuver by disconnecting patients from ventilators and allowing them to exhale to atmospheric pressure (40). The authors showed how the elastance-derived end-inspiratory Pl was closely correlated with the release-derived value; while it did not require patients to be disconnected from the ventilator, the elastance-derived Pl can then be easily used as an estimate for end-inspiratory stress.
Direct measurement
With this method, proposed by Talmor and colleagues (17,39) Pl is simply calculated as the absolute difference between airway and Pes:
Pl = Paw − Pes.
As a consequence of the assumption that actual values of Pes reflect absolute values of Ppl, many patients show negative end-expiratory Pl. This has been suggested to reflect lung regions at risk of cyclic tidal opening/closing or lung collapse; the negative value of Pl is but a mathematical consequence of the calculation method, and it may depend on proximal airway closure during exhalation, alveolar flooding or it may be due to regional variations in Ppl in inhomogeneous lungs. The crude calculation of absolute Pl has raised doubts about its reliability, as several confounding may affect the actual value of Pes, as extensively stated earlier.
In an attempt to reconcile these conflicting approaches, an experimental study was recently carried out. Yoshida et al. measured Pes across a range of PEEP levels, together with directly measured Ppl in non-dependent and dependent pleural regions, both in a swine model of lung injury and in human cadavers (45). The authors also computed Pl with both the directly-measured and the elastance-derived method, and found how both methods reasonably reflect the “true” Pl, although in different lung regions.
Indeed, directly-measured Pl tightly mirrored “true” Pl in the regions close to the esophageal balloon (i.e., dependent-to-middle lung). Thus, directly-measured end-expiratory Pes may potentially be useful to tailor the level of PEEP needed open atelectasis in dependent regions. On the other hand, end-inspiratory Pl, as obtained with the elastance-derived method, was found to reflect the “true” local Pl in the non-dependent areas. Then, with this method, Pl may be used to find the highest level of inspiratory stress, and it may be used as a target to reduce VILI.
Transpulmonary pressure assessment during controlled mechanical ventilation
Since Paw and tidal volume have proven inadequate surrogates for lung stress and strain (18), the use of Pl has been proposed as a better means of adjusting the settings of mechanical ventilation. Despite the sound pathophysiological rationale, clinical studies evaluating the efficacy of such an approach are still lacking. During controlled mechanical ventilation, Pl has been used with two different aims: help clinicians to provide a sufficient level of PEEP to avoid derecruitment and atelectrauma, or to provide a better estimate of lung distending pressure, then reducing the risk of VILI. These two approaches have been pursued with two different methods for the estimation of Pl, namely the directly-measured method and the elastance-derived method, respectively.
An influential trial by Talmor et al. analysed the effect of setting PEEP according to the measurement of end-expiratory Pl in patients with ARDS (39). 61 patients were randomized to a standard FiO2/PEEP table (46) or a strategy based on PEEP increase until directly-measured end-expiratory Pl was within a positive range (0–10 cmH2O). An average 88 mmHg higher PaO2/FiO2 ratio and decreased Ers were found in the Pes-guided group. The level of PEEP in the Pes-guided group was significantly higher than in the control group, with no signs of hemodynamic compromise and an end-inspiratory Pl always lower than the limit of 25 cmH2O. However, in a subsequent study, Chiumello et al. found how the setting of PEEP based on such directly-measured approach was not related to thoracic CT-scan lung recruitability, nor with lung weight or the severity of the disease, casting doubts about the assumptions underlying the direct method for Pl estimation (47).
On the other hand, Grasso et al. evaluated whether monitoring lung distending pressure by the elastance-derived end-inspiratory Pl (as opposed to the use of plateau Paw) might allow the clinicians to safely increase the level of PEEP with the aim of improving oxygenation and avoiding the unnecessary use of extracorporeal support in patients with ARDS from H1N1 influenza and refractory hypoxemia (19). Indeed, since Paw may depend upon chest wall mechanics and patient respiratory muscle activity, Grasso and colleagues hypothesized that, in selected patients with severe ARDS, the end-inspiratory Pl could be low enough to allow safe increases of PEEP and lead to improved lung recruitment when a relatively large proportion of Paw was dissipated against a stiff chest wall, despite the presence of a high Paw. The authors set a target end-inspiratory Pl of 25 cmH2O in 14 patients. While 7 patients had end-inspiratory Pl >27 cmH2O, and all underwent ECMO, the other 7 had an average Pl of about 16.6 cmH2O. In this group of patients, an average 4.4 cmH2O increase of PEEP (from 17.9 to 22.3 cmH2O) lead to improved oxygenation and prevented the use of extracorporeal support.
Despite both using Pl as an estimate of the pressure applied to the lung, the two approaches differ significantly in their assumptions, as we highlighted in the previous section. The approach used by Talmor utilizes directly-measured Pl to adjust PEEP in order to keep end-expiratory Pl >0 cmH2O (39). Grasso used the elastance-derived method to target an end-inspiratory Pl of 25 cmH2O (19). Hence, application of both strategies to the same patients may yield different results. Two recent studies directly compared the two methods for Pl estimation in the same patients in a cross-over fashion. Gulati et al. compared the direct and the elastance-derived method with a target of end-inspiratory Pl of 26 cmH2O. The authors found incompatible results between the two approaches, to the extent that differences in the estimate of Ppl could be as high as 10 cmH2O for a given patient. Moreover, the optimal levels of PEEP recommended by the two methods were discordant and unrelated, so that the suggested changes in PEEP moved into the opposite direction in up to a third of patients (48). Similarly, Chiumello et al. compared the directly-measured end-expiratory Pl and that obtained by the release method in 44 patients with ARDS (40). Again, the two values of Pl were significantly different and unrelated. Moreover, the value of Pes at atmospheric pressure was not related to the extent of lung consolidation or recruitability as assessed by CT scan, nor to the degree of hypoxemia or the value of chest wall elastance. On the other side, the end-inspiratory Pl estimated with either the elastance-derived or the release-derived methods showed a good correlation. In a subsequent study (47), the same authors found how in a cohort of 51 patients with ARDS with different severity of the disease, the PEEP levels suggested from targeting an end-expiratory Pl >0 were unrelated to lung recruitability (as assessed by lung CT scan) and similar for all patients despite the severity of their disease.
In summary, although clinically feasible, the calculation of Pl from Pes as a strategy for tailoring ventilator support at the individual level still needs to be supported by further trials. Since every method for the estimation of Pl is based on assumptions, also the choice of which method to use should be guided by further investigations.
Transpulmonary pressure assessment during spontaneous/assisted breathing
During assisted modes of mechanical ventilation, the total WOB is shared between the mechanical ventilator and patient muscles. Then, according to the equation of motion, the pressure required for lung inflation is the sum of the pressure generated by the respiratory muscles (Pmusc) and that applied by the ventilator to the airway (Paw). During such a breathing, active contraction of the diaphragm and other inspiratory muscles triggers the mechanical breaths, and the total pressure developed by the muscles depends upon both the respiratory drive and the strength of the muscles. As a consequence, Paw does not mirror Pl, since the downward displacement of the diaphragm generates a negative Ppl swing. Since Pl is the difference between the positive pressure provided by the ventilator, minus the negative pressure generated by the respiratory muscles, Pl is in general even higher than Paw. Figure 4 shows an example of the contribution of positive Paw and negative Pes to the Pl during assisted breathing.

Indeed, in the presence of vigorous spontaneous breathing efforts, high negative Ppls are generated, leading to elevated Pl despite normal-appearing Paw (49). Moreover, patient-ventilator interaction may sometimes be difficult to assess when only the standard monitoring of tidal volume and Paw is used. Measurement of Pes may allow the clinician to assess patient’s real respiratory effort, patient-ventilator (a)synchrony, the presence of intrinsic PEEP and the calculation of patient and ventilator contribution to the total WOB. Assessment of Pes may guide the titration of the level of ventilator support at the individual level, as well as to monitor the level of fatigue during a weaning trial and even to predict the failure of the weaning process. Monitoring Pes allows to calculate the pressure-time product of Pes, an index related to the metabolic cost of breathing; during a trial of spontaneous breathing, this index significantly increased only in patients who then failed weaning (50). The tidal swing in Pes, used as an index respiratory effort, was shown to progressively increase as patients failed a weaning trial (51), and it allowed better discrimination between patients who failed and those who succeeded the trial than conventional index such as the rapid shallow breathing index (52).
Recently, Bellani et al. conducted a study aiming at comparing the tidal change in transpulmonary pressure during assisted breathing and controlled ventilation, after matching for similar conditions of airflow and volume, in a group of patients undergoing different levels of pressure support ventilation followed by a phase of controlled mechanical ventilation (12). The authors demonstrated how, for a given flow and tidal volume (assuming unchanged mechanical properties of the system), tidal changes of Pl were similar between the different conditions of support and regardless of the level of inspiratory effort, whereas the absolute value of airway and Pes were different, thus highlighting the importance of measurement of Pes during assisted modes of breathing. In fact, preservation of spontaneous breathing was shown to be associated with different beneficial effects, such as improved hemodynamics (53), a better ventilation-to-perfusion matching (54), and a reduced extent of muscle atrophy (55). However, other studies found how spontaneous breathing efforts could potentially worsen lung injury (49,56,57), likely because of the effects of negative intrathoracic pressure (which may lead to interstitial edema), generation of unsafe stress and excessively elevated Pl and loss of control over tidal volume. In an experimental model of ARDS, allowing spontaneous breathing had beneficial effects in terms of lung recruitment only in case of mild lung injury, whereas it worsened lung injury in more severely ill animals, likely because of the development of injuriously high transpulmonary pressure (57). In this regard, even if no studies have so far been conducted in patients, end-inspiratory Pl should likely be kept <20–25 cmH2O, which is the upper limit of physiological range (20).
In summary, monitoring Pes during assisted modes of ventilation is highly relevant. First, estimation of Pl may prove invaluable to detect the harm of spontaneous efforts; second, it may allow individual titration of ventilator support to prevent diaphragm injury and accelerate liberation from ventilation.
Conclusions
Despite data showing its relevance, assessment of Pl and Pes monitoring is still hardly used in critical care medicine. This may partially be due to technical issues, such as proper placement of the esophageal catheter, and because of the difficult interpretation of the measurements. However, a strong pathophysiological rationale, and an increasing amount of clinical evidence convincingly show how this technique may provide an invaluable insight for the management of critically ill patients, both during controlled mechanical ventilation (to allow partitioning between lung and chest wall) and during assisted ventilation (to assess the contribution of respiratory muscles and the interaction with the ventilator).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gattinoni L, Quintel M. Is mechanical ventilation a cure for ARDS? Intensive Care Med 2016;42:916-7. [Crossref] [PubMed]
- Nash G, Blennerhassett JB, Pontoppidan H. Pulmonary lesions associated with oxygen therapy and artifical ventilation. N Engl J Med 1967;276:368-74. [Crossref] [PubMed]
- Avignon PD, Hedenstrom G, Hedman C. Pulmonary complications in respirator patients. Acta Med Scand Suppl 1956;316:86-90. [PubMed]
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126-36. [Crossref] [PubMed]
- Tonetti T, Vasques F, Rapetti F, et al. Driving pressure and mechanical power: new targets for VILI prevention. Ann Transl Med 2017;5:286. [Crossref] [PubMed]
- Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998;157:294-323. [Crossref] [PubMed]
- Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 2007;175:160-6. [Crossref] [PubMed]
- King EG. Monitoring of ventilation in the critically ill. Can Med Assoc J 1977;117:991-2. [PubMed]
- Mutoh T, Lamm WJ, Embree LJ, et al. Volume infusion produces abdominal distension, lung compression, and chest wall stiffening in pigs. J Appl Physiol (1985) 1992;72:575-82. [PubMed]
- Malbrain ML, Chiumello D, Pelosi P, et al. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med 2004;30:822-9. [Crossref] [PubMed]
- Cortes-Puentes GA, Keenan JC, Adams AB, et al. Impact of Chest Wall Modifications and Lung Injury on the Correspondence Between Airway and Transpulmonary Driving Pressures. Crit Care Med 2015;43:e287-95. [Crossref] [PubMed]
- Bellani G, Grasselli G, Teggia-Droghi M, et al. Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study. Crit Care 2016;20:142. [Crossref] [PubMed]
- Cortes GA, Marini JJ. Two steps forward in bedside monitoring of lung mechanics: transpulmonary pressure and lung volume. Crit Care 2013;17:219. [Crossref] [PubMed]
- Gattinoni L, Pelosi P, Suter PM, et al. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med 1998;158:3-11. [Crossref] [PubMed]
- Pelosi P, Cereda M, Foti G, et al. Alterations of lung and chest wall mechanics in patients with acute lung injury: effects of positive end-expiratory pressure. Am J Respir Crit Care Med 1995;152:531-7. [Crossref] [PubMed]
- Gattinoni L, Chiumello D, Carlesso E, et al. Bench-to-bedside review: chest wall elastance in acute lung injury/acute respiratory distress syndrome patients. Crit Care 2004;8:350-5. [Crossref] [PubMed]
- Talmor D, Sarge T, O'Donnell CR, et al. Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med 2006;34:1389-94. [Crossref] [PubMed]
- Chiumello D, Carlesso E, Cadringher P, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med 2008;178:346-55. [Crossref] [PubMed]
- Grasso S, Terragni P, Birocco A, et al. ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med 2012;38:395-403. [Crossref] [PubMed]
- Mauri T, Yoshida T, Bellani G, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med 2016;42:1360-73. [Crossref] [PubMed]
- Cherniack RM, Farhi LE, Armstrong BW, et al. A comparison of esophageal and intrapleural pressure in man. J Appl Physiol 1955;8:203-11. [Crossref] [PubMed]
- Mead J, Mc IM, Selverstone NJ, et al. Measurement of intraesophageal pressure. J Appl Physiol 1955;7:491-5. [Crossref] [PubMed]
- Mead J, Gaensler EA. Esophageal and pleural pressures in man, upright and supine. J Appl Physiol 1959;14:81-3. [Crossref] [PubMed]
- Milic-Emili J, Mead J, Turner JM, et al. Improved Technique for Estimating Pleural Pressure from Esophageal Balloons. J Appl Physiol 1964;19:207-11. [Crossref] [PubMed]
- Gibson GJ, Pride NB. Lung distensibility. The static pressure-volume curve of the lungs and its use in clinical assessment. Br J Dis Chest 1976;70:143-84. [Crossref] [PubMed]
- Hedenstierna G. Esophageal pressure: benefit and limitations. Minerva Anestesiol 2012;78:959-66. [PubMed]
- Knowles JH, Hong SK, Rahn E. Possible errors using esophageal balloon in determination of pressure-volume characteristics of the lung and thoracic cage. J Appl Physiol (1985) 1959;14:525-30.
- Milic-Emili J, Mead J, Turner JM. Topography of Esophageal Pressure as a Function of Posture in Man. J Appl Physiol 1964;19:212-6. [Crossref] [PubMed]
- Washko GR, O'Donnell CR, Loring SH. Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J Appl Physiol (1985) 2006;100:753-8. [PubMed]
- Agostoni E, D'Angelo E, Bonanni MV. Topography of pleural surface pressure above resting volume in relaxed animals. J Appl Physiol 1970;29:297-306. [Crossref] [PubMed]
- Agostoni E, D'Angelo E, Bonanni MV. The effect of the abdomen on the vertical gradient of pleural surface pressure. Respir Physiol 1970;8:332-46. [Crossref] [PubMed]
- Loring SH, O'Donnell CR, Behazin N, et al. Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol (1985) 2010;108:515-22. [PubMed]
- Lai-Fook SJ, Rodarte JR. Pleural pressure distribution and its relationship to lung volume and interstitial pressure. J Appl Physiol (1985) 1991;70:967-78. [PubMed]
- de Chazal I, Hubmayr RD. Novel aspects of pulmonary mechanics in intensive care. Br J Anaesth 2003;91:81-91. [Crossref] [PubMed]
- Cressoni M, Chiurazzi C, Gotti M, et al. Lung inhomogeneities and time course of ventilator-induced mechanical injuries. Anesthesiology 2015;123:618-27. [Crossref] [PubMed]
- Graf J, Formenti P, Santos A, et al. Pleural effusion complicates monitoring of respiratory mechanics. Crit Care Med 2011;39:2294-9. [Crossref] [PubMed]
- Pelosi P, D'Andrea L, Vitale G, et al. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med 1994;149:8-13. [Crossref] [PubMed]
- Pelosi P, Goldner M, McKibben A, et al. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 2001;164:122-30. [Crossref] [PubMed]
- Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008;359:2095-104. [Crossref] [PubMed]
- Chiumello D, Cressoni M, Colombo A, et al. The assessment of transpulmonary pressure in mechanically ventilated ARDS patients. Intensive Care Med 2014;40:1670-8. [Crossref] [PubMed]
- Gattinoni L, Cressoni M, Chiumello D, et al. Transpulmonary Pressure Meaning: Babel or Conceptual Evolution? Am J Respir Crit Care Med 2017;195:1404-5. [Crossref] [PubMed]
- Chiumello D, Colombo A, Algieri I, et al. Effect of body mass index in acute respiratory distress syndrome. Br J Anaesth 2016;116:113-21. [Crossref] [PubMed]
- Higgs BD, Behrakis PK, Bevan DR, et al. Measurement of pleural pressure with esophageal balloon in anesthetized humans. Anesthesiology 1983;59:340-3. [Crossref] [PubMed]
- Polese G, Rossi A, Appendini L, et al. Partitioning of respiratory mechanics in mechanically ventilated patients. J Appl Physiol (1985) 1991;71:2425-33. [PubMed]
- Yoshida T, Amato MBP, Grieco DL, et al. Esophageal Manometry and Regional Transpulmonary Pressure in Lung Injury. Am J Respir Crit Care Med 2018;197:1018-26. [Crossref] [PubMed]
- Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Chiumello D, Cressoni M, Carlesso E, et al. Bedside selection of positive end-expiratory pressure in mild, moderate, and severe acute respiratory distress syndrome. Crit Care Med 2014;42:252-64. [Crossref] [PubMed]
- Gulati G, Novero A, Loring SH, et al. Pleural pressure and optimal positive end-expiratory pressure based on esophageal pressure versus chest wall elastance: incompatible results. Crit Care Med 2013;41:1951-7. [Crossref] [PubMed]
- Yoshida T, Uchiyama A, Matsuura N, et al. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med 2012;40:1578-85. [Crossref] [PubMed]
- Laghi F, Cattapan SE, Jubran A, et al. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 2003;167:120-7. [Crossref] [PubMed]
- Jubran A, Tobin MJ. Passive mechanics of lung and chest wall in patients who failed or succeeded in trials of weaning. Am J Respir Crit Care Med 1997;155:916-21. [Crossref] [PubMed]
- Jubran A, Grant BJ, Laghi F, et al. Weaning prediction: esophageal pressure monitoring complements readiness testing. Am J Respir Crit Care Med 2005;171:1252-9. [Crossref] [PubMed]
- Putensen C, Zech S, Wrigge H, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 2001;164:43-9. [Crossref] [PubMed]
- Putensen C, Mutz NJ, Putensen-Himmer G, et al. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1999;159:1241-8. [Crossref] [PubMed]
- Futier E, Constantin JM, Combaret L, et al. Pressure support ventilation attenuates ventilator-induced protein modifications in the diaphragm. Crit Care 2008;12:R116. [Crossref] [PubMed]
- Yoshida T, Torsani V, Gomes S, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med 2013;188:1420-7. [Crossref] [PubMed]
- Yoshida T, Uchiyama A, Matsuura N, et al. The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit Care Med 2013;41:536-45. [Crossref] [PubMed]


