Which criteria should we use to evaluate the efficacy of immune-checkpoint inhibitors?
Immune-checkpoint inhibitors (ICI) have innovated the treatment of many different types of advanced cancer. Two important distinctions between ICI and other modalities are durable response (DR) and pseudoprogression (1,2).
DR refers to long lasting tumor control, which is unavailable with conventional modalities. DR has been reported to occur in 10–20% of patients treated with ICI (3,4), and in some patients who achieve DR, relapse is not observed after treatment discontinuation (3).
Despite the remarkable effect of ICI in some patients, the majority of patients do not see a benefit from ICI. Selecting patients who will benefit from ICI is a major issue in the application of this treatment. Several biomarkers have been investigated to select patients before treatment. Programmed death ligand-1 (PD-L1) and tumor mutation burden are useful, but not perfect, markers (5). Other biomarkers (e.g., the lymphocyte/neutrophil ratio, lactate dehydrogenase, and carcinoembryonic antigen) have been explored, but show limited predictive value (6-8). Hence, determining criteria to assess the benefit of ICI during treatment is important.
A few patients treated with ICI respond with an initial increase in total tumor volume, a phenomenon termed “pseudoprogression” (9). The existing standard criteria for evaluating response in cancer clinical trials are the World Health Organization (WHO) criteria and the Response Evaluation Criteria in Solid Tumors (RECIST) (10,11); however, neither is adequately equipped to appropriately evaluate pseudoprogression. Because they cannot distinguish pseudoprogression from progressive disease (PD). Three new criteria have been proposed to solve this problem (Table 1). Wolchok et al. reported the immune-related response criteria (irRC), an improved version of the WHO criteria (2). IrRC requires evaluation of the two-dimensional tumor burden, which requires more effort than one-dimensional evaluation (12). Nishino et al. reported the immune-related response evaluation criteria in solid tumors (irRECIST), which combines the features of irRC and RECIST. IrRECIST requires only one-dimensional measurement and need to confirm to judge PD (13). IrRECIST has not always been applied in the same way, leading to concerns about the comparability of results across studies (14). Seymour et al. reported the immune response evaluation criteria in solid tumors (iRECIST), an improved version of RECIST 1.1 (14). In iRECIST, the measurements of the new lesion(s) are not incorporated into the tumor burden, which is the main difference from irRECIST. IRECIST is developed by consensus, and the relationship with prognosis has not been clearly evaluated (14).
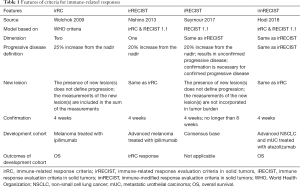
Full table
The recent study published in the Journal of Clinical Oncology by Hodi et al. proposed the immune-modified response evaluation criteria in solid tumors (imRECIST) (15). They developed the criteria to evaluate the outcomes of patients treated with atezolizumab, which was reported for the first time at the American Society of Clinical Oncology Annual Meeting (16). They evaluated the relationship of imRECIST and overall survival (OS) in non-small cell lung cancer (NSCLC) and metastatic urothelial carcinoma, and the progression pattern in renal cell carcinoma and melanoma. ImRECIST is almost identical to irRECIST; indeed, the authors overlap. The novelties of imRECIST include a more detailed definition of progression-free survival and evaluation of the relationship of prognosis in several cancers.
In clinical trials, surrogate endpoints such as overall response rate or progression-free survival are evaluated for the purpose of predicting OS, which is the ultimate endpoint (17). Several studies have reported a relationship between the criteria and OS (Table 2) (14,18-20). They showed the difference between the overall response and OS by Kaplan-Meier curve in several cancers. Currently, iRECIST and imRECIST are the most promising criteria with respect to convenience. Because we have limited data in regard to tumor type and the evaluated settings in advanced cancers, we cannot draw conclusions as to which criteria are superior.

Full table
One limitation for use of these criteria is that all the criteria were developed for use in clinical trials. In general patient care, we should prudent to consulting these criteria to stop administration of ICI.
Further evaluation to clarify the difference of necessary effort, predictive accuracy in other types of cancers, and other treatment sequences (e.g., neo-adjuvant) are warranted.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56-61. [Crossref] [PubMed]
- Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clin Cancer Res 2009;15:7412-20. [Crossref] [PubMed]
- Robert C, Ribas A, Hamid O, et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J Clin Oncol 2018;36:1668-74. [PubMed]
- Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: Two-year outcomes from two randomized, open-label, phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924-33. [Crossref] [PubMed]
- Rizvi H, Sanchez-Vega F, La K, et al. Molecular Determinants of Response to Anti–Programmed Cell Death (PD)-1 and Anti–Programmed Death-Ligand (PD-L)-Ligand 1 Blockade in Patients With Non–Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J Clin Oncol 2018;36:633-41. [Crossref] [PubMed]
- Kataoka Y, Hirano K, Narabayashi T, et al. Carcinoembryonic Antigen as a Predictive Biomarker of Response to Nivolumab in Non-small Cell Lung Cancer. Anticancer Res 2018;38:559-63. [PubMed]
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. [Crossref] [PubMed]
- Diem S, Kasenda B, Spain L, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer 2016;114:256-61. [Crossref] [PubMed]
- Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 2018;88:38-47. [Crossref] [PubMed]
- Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981;47:207-14. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Nishino M, Gargano M, Suda M, et al. Optimizing immune-related tumor response assessment: Does reducing the number of lesions impact response assessment in melanoma patients treated with ipilimumab? J Immunother Cancer 2014;2:17. [Crossref] [PubMed]
- Nishino M, Giobbie-Hurder A, Gargano M, et al. Developing a Common Language for Tumor Response to Immunotherapy: Immune-Related Response Criteria Using Unidimensional Measurements. Clin Cancer Res 2013;19:3936-43. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Hodi FS, Ballinger M, Lyons B, et al. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J Clin Oncol 2018;36:850-8. [Crossref] [PubMed]
- Mazieres J, Fehrenbacher L, Rittmeyer A, et al. Non-classical response measured by immune-modified RECIST and post-progression treatment effects of atezolizumab in 2L/3L NSCLC: Results from the randomized phase II study POPLAR. Clin Oncol 2016;15:9032.
- Blumenthal GM, Zhang L, Zhang H, et al. Milestone analyses of immune checkpoint inhibitors, targeted therapy, and conventional therapy in metastatic non–small cell lung cancer trials: A meta-analysis. JAMA Oncol 2017;3:e171029. [Crossref] [PubMed]
- Khoja L, Kibiro M, Metser U, et al. Patterns of response to anti-PD-1 treatment: An exploratory comparison of four radiological response criteria and associations with overall survival in metastatic melanoma patients. Br J Cancer 2016;115:1186-92. [Crossref] [PubMed]
- Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with Pembrolizumab. J Clin Oncol 2016;34:1510-7. [Crossref] [PubMed]
- Kataoka Y, Hirano K, Narabayashi T, et al. Concordance between the response evaluation criteria in solid tumors version 1.1 and the immune-related response criteria in patients with non-small cell lung cancer treated with nivolumab: a multicenter retrospective cohort study. Cancer Chemother Pharmacol 2018;81:333-7. [Crossref] [PubMed]


