Tau in neurodegenerative disease
Introduction
Neurodegenerative diseases with abnormal tau inclusions are referred to as tauopathies, including Alzheimer disease (AD), frontotemporal dementia with parkinsonism-17 (FTDP-17), Pick disease (PiD), progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD). AD, the most common cause of dementia, is diagnosed by extracellular plaques containing Aβ peptides and intracellular neurofibrillary tangles (NFTs) from hyperphosphorylated, insoluble and filamentous tau proteins in neuropathology (1). The discovery of mutations in the microtubule-associated protein tau (MAPT) gene in patients with FTDP-17 also contributes to a better understanding of the dysfunction tau as a cause of diseases (2). There are also numerous tau-related neurodegenerative diseases with various clinical and pathological features. However, the tau pathology is a critical event throughout the neurodegenerative process. Although recent substantial progress has been made in the tau pathology of tauopathies, the mechanisms underlying tau-induced neurodegeneration remain unclear.
The role of tau in neurodegenerative disease is now a hot research area. Here, we review and summarize the biochemical properties of tau protein and the pathogenesis underlying tau-induced neurodegenerative diseases. Meanwhile, we also consider the tau-related biomarkers and ongoing tau-targeted strategies for therapeutic modulation.
Tau protein
Molecular genetics, expression and structure of tau
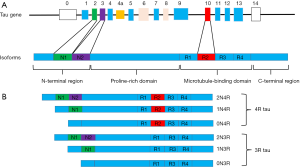
The MAPT is encoded by a single gene, MAPT, which comprises 16 exons on chromosome 17q21 (Figure 1). The human tau protein is composed of four areas: an N-terminal region, a proline-rich domain, a microtubule-binding domain (MBD), and a C-terminal projection region (3). There are six main isoforms of tau expressed in the human brain, which are generated by alternative splicing around the N-terminal region [exon 2 (E2) and E3] and MBD (E10) (4). They differ from each other depending on the number of 29-aminoacid inserts, and the presence of three microtubule binding repeat sequences (3R) or 4R (5). The difference is that 4R tau has four microtubule binding repeat sequences due to the inclusion of E10, which is not included in 3R tau. In the normal human brain, there are equal amounts of 3R and 4R tau, but this ratio is often changed in neurodegenerative diseases.
Biological functions of tau
As a microtubule-associated protein, tau is supposed to be essential for the regulation of microtubule structure and dynamics by binding to the microtubule surface through the MBD and adjacent regions. Tau may regulate axonal transport by binding to the microtubule. Based on the graded distribution of the tau along the axon, the cargo can be detached from motor proteins, increasing axonal transport efficiency (6,7). With tau-knockout mice having been widely applied in the research, recent reports have revealed several novel functions of tau, such as maintenance of the integrity of genomic DNA (8,9), regulation of neuronal activity (10-13), neurogenesis (14,15), iron export (16) and long-term depression (LTD) (17,18). Understanding the additional functions of tau is not only essential to elucidate the tau pathogenesis, but is also necessary to ascertain the tau-based therapeutic strategy.
Tau in neurodegenerative diseases
Tau in Alzheimer’s disease
With the aging of global population, AD is becoming one of the greatest health-care challenges in the twenty-first century (19). Over the past decade, significant advances have been reported in understanding the roles of tau in physiology and pathology.
Tau aggregation in AD
As a natively unfolded protein, tau presents little tendency for aggregation in structure. Evidence has revealed that the tau molecule might be prone to conformation change to make a paperclip-like structure in the presence of N-terminal, C-terminal and MBD (20). The paperclip-like shape might prevent from tau aggregation. Once this structure is broken, for example, hyperphosphorylation, these inhibitory domains of tau molecule will be neutralized and then tau protein self-assembles into tangles of PHF (21).
Tau aggregation could be affected by post-translational modifications. In particular, phosphorylation, as a common characteristic of AD, has been assumed to drive tau aggregation. Other post-translational modifications also show inhibition or promotion effects on tau aggregation, including O-GlcNAcylation (22), deamidation, isomerization (23), methylation (24), etc.
Experimental investigation shows that tau aggregation is essential for tau-induced toxicity. Tau aggregation may decrease levels of soluble functional tau, sequester other cell components or hinder axonal transport, finally resulting in neurodegeneration.
Tau phosphorylation and other post-translational modifications in AD
Generally, an appropriate amount of phosphorylation is necessary for the realization of physiological functions of tau, whereas the hyperphosphorylated state reduces its biological activity.
The state of phosphorylation of the tau protein is mainly modulated by various protein kinases and phosphatases. Tau is phosphorylated by several protein kinases, which are classified into three groups including proline-directed protein kinases (PDPKs), non-PDPKs and tyrosine kinases. It has been found that there were multiple combinations of PDPKs and non-PDPKs in the AD patients’ brain, implying that they could be involved in the phosphorylation of tau (25). Besides, tau protein could also be phosphorylated by tyrosine kinases including the Src family kinases (SFKs), FYN and the ABL family kinases (26). What’s more, tau hyperphosphorylation could also arise from the inhibition of phosphatases, including protein phosphatase1 (PP1), PP2A, PP2B, PP2C and PP5 (26), among which PP2A is the major phosphatase and accounts for more than 70% of the tau phosphatase activity in human brain (27). PP2A regulates the phosphorylation of tau directly or by regulating several tau protein kinases activity (28). It was observed that the PP2A activity has declined by 50% in brain of AD patients (27,29,30).
Hyperphosphorylation of tau-induced pathology is primarily through damaging the normal functions of tau. The affinity of tau to microtubules might be weakened by the hyperphosphorylation of tau in the repeat domain, causing microtubule instability and disassembly and then promoting tau self-aggregation (26). Other mechanisms of phosphorylated tau-induced toxicity have been investigated intensely already. For example, the phosphorylation of tau might induce tau missorting from axons to postsynaptic terminals or dendrites (31-33), alter its degradation and truncation (34,35), and affect the connection of tau with its interacting partners (36), leading to neurodegeneration in the end.
Apart from phosphorylation, tau is also subject to other post-translational modifications, such as tau acetylation (37-39), N-glycosylation (40), O-GlcNAcylation (41), nitration (42), ubiquitination (43,44), sumoylation (45), isomerization (46,47), and truncation (48,49). Although the exact roles of these modifications remain unclear so far, the importance of each tau post-translational modification during the pathological process could never be overlooked.
Tau oligomers and propagation of tau pathology in AD
Although NFT is a pathologic hallmark of AD, growing evidence shows that NFT formation may not be necessary for neurodegeneration. In wild-type or transgenic mice, synaptic loss, memory deficits and electrophysiological impairments develop before or without any eventual NFT formation (50). These data highlighted that neuronal toxicity might be induced by smaller, soluble tau species, possibly oligomers. Tau oligomers have been detected in brain tissues of AD patients and its level shows a relation with the degree of memory deficits (51). However, the role of tau oligomers still remains controversial. Based on the heterogeneity of oligomer preparations and different assay methods, some studies found that the tau oligomers made from tau aggregation would induce neuronal loss and cognitive dysfunction (52,53).
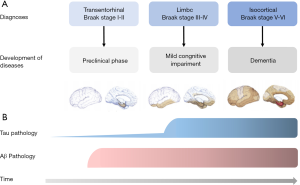
It is beyond doubt that the tau pathology could not be restricted to intracellular environment. Based on the Braak staging theory, tau pathology is firstly detected in the transentorhinal and entorhinal regions (stages I and II). Afterward, it was also observed in the hippocampus (stages III and IV) and was eventually widespread in the cortex (stages V and VI) (54) (Figure 2). Evidence shows that non-fibrillar oligomers of hyperphosphorylated tau are the endogenous form of tau involved in propagation in primary neurons (55). It is assumed that the pathological spread of tau oligomers (seeds) occurs through endocytosis, and trans-synaptic pathway by exosomes or synaptic vesicles transferring between neurons (56-59). Once drawn by postsynaptic neurons, tau seeds trigger tau aggregation subsequently. Now the propagation pattern of trans-synaptic spread has been widely accepted.
Tau and Aβ
AD is defined by the presence of amyloid plaques and neuronal tangles. There is considerable evidence suggesting that Aβ and tau play pivotal roles in the disease process. They could also influence and regulate the toxic effects of each other, whereas the regulatory mechanism of their interaction has not been identified so far.
Several experiments indicate that the formation of Aβ is considered as the trigger, or even the driver of the tau pathology, despite the fact that it’s formed after the tau deposits. In neurons which were acutely exposed to Aβ, Aβ initiated tau-mediated neurotoxicity by facilitating tau phosphorylation (60) and directing pathogenic tau species into dendritic spines (33), leading to spine collapse and dendritic degeneration. Evidence shows that tau protein is essential for Aβ-induced neurotoxicity. Tau reduction could effectively protect the brain against Aβ by inhibiting neuronal over-excitation (61), excitotoxicity, memory deficits and premature mortality (62). According to the evidence, it is suggested that tau may mediate Aβ toxicity by modulating the tyrosine kinase (Fyn), which provides a link between Aβ and tau. Ittner et al. have demonstrated that knocking out tau or expressing truncated tau in transgenic mice disrupts postsynaptic targeting of Fyn, perturbs the NMDA receptor/PSD-95 interaction and then ameliorates Aβ toxicity (62). To elucidate the role of the Aβ-Fyn-tau triad in neuronal dysfunction might generate therapeutic benefit in AD (63).
Tau in frontotemporal dementia (FTD)
FTD is a collective term referring to a heterogeneous group of sporadic and familial neurodegenerative disorders that cause selective atrophy of the frontal and temporal lobes. Some tauopathies histopathologically show tau inclusions could play a part in the clinical development of FTD, for instance, FTDP-17 and PiD.
Tau gene mutations in FTDP-17
FTDP-17 is clinically defined as a neurodegenerative syndrome manifested by behavioral, cognitive and motor disturbances. To date, over 50 mutations have been confirmed in exonic and intronic domains. They can be classified into two categories, including missense mutations and splicing mutations, which affect the sequence of tau and influence the correlation proportion of different tau isoforms. It has been found that most of missense mutations localized in the MBD or the adjacent region can weaken the ability of tau to interact with microtubules and increase tendency for aggregation (64,65). In addition, missense mutations in C-terminal domain inhibit tau binding to microtubule and therefore promote microtubule assembly less efficiently, whereas axonal transport might be disordered by the disruption of the binding of tau to the dynactin complexin the N-terminal domain (66). Most splicing mutations occur in intron 10 and alter the ratio of 4R tau to 3R tau by increasing the inclusion of E10 (repeat R2), which results in the overproduction and the assembly of 4R tau into filaments. Comparatively speaking, 4R tau is a more favorable substrate for hyperphosphorylation than 3R-tau (67). In conclusion, tau mutations and an altered 3R:4R ratio can lead to the loss of normal function or a toxic gain of function, eventually leading to neurodegeneration.
Tau in PiD
Among a variety of the causes of frontotemporal dementia, PiD is rare, accounting for a small proportion of all dementia cases (68). The most common clinical manifestation is behavioral variant frontotemporal dementia (bvFTD) with frontotemporal atrophy (69). Besides, apraxia (70) and progressive nonfluent aphasia (PNFA) (71) have also been reported. The abovementioned clinical presentation could be determined by the distribution of pathologies. Pathologically, PiD is characterized by asymmetric, circumscribed cortical atrophy, which is associated with neuronal loss, gliosis and distinctive neuronal inclusions (Pick bodies). According to a classification scheme, PiD could be classified into three types, including type A (classic PiD, demonstrating both Pick bodies and Pick cells), type B (CBD, demonstrating only Pick cells) and type C (dementia lacking distinctive histology, exhibiting neither Pick bodies nor Pick cells) (72).
PiD is usually considered as a sporadic disease. However, the mutations in the MAPT in the majority of familial PiD cases whose cause pathology and clinical presentation are indistinguishable from sporadic PiD (73). Recent studies have developed a novel 3R mutant tau transgenic model to promote the understanding about the natural history, progression and therapy of PiD (74). Moreover, a novel missense mutant in E12 of MAPT (p.Q336H) has been found to cause PiD, possibly by increasing selective aggregation of 3R-tau isoforms (75).
Tau in PSP and CBD
PSP and CBD, as rare progressive neurodegenerative diseases, are often classified within the spectrum of 4R tauopathy disease. Pathologically, both CBD and PSP are characterized by the accumulation of tau-positive inclusions that contain predominant 4R tau in neuronal and glial cells. The neuronal lesion is the pretangles and tangles. Oligodendroglial lesion is the coiled bodies both in PSP and CBD. The hallmark astrocyte lesion is the tufted astrocyte and the astrocytic plaque in PSP and CBD, respectively (76,77). The morphologic features of cells lesion have been used to distinguish PSP and CBD (78-80) yet with no specific markers to be detected.
Although there is little to know about the genetics of CBD and PSP, several studies have proven that they have a common genetic underpinning. H1c, which is a common occurring haplotype in the tau gene, could contribute to disease pathogenesis probably through the impact upon the interplay of tau isoforms (81). In addition, there was an overlap of the clinical and pathologic features of patients with mutations in MAPT between PSP (82) and CBD (83).
Tau in other tauopathies
Varied clinical manifestations and pathological features have been shown by other tauopathies with tau deposits, such as Down’s syndrome (DS), argyrophilic grain disease (AGD) and Parkinson’s disease complex of Guam (PDC Guam). There are also numerous tau-related neurodegenerative diseases with various clinical and pathological features. However, tau pathology is a critical event throughout neurodegenerative process.
Tau as a biomarker for neurodegenerative diseases
As mentioned above, the levels of tau in patients of neurodegenerative diseases are correlated with neuronal dysfunction and degeneration in the brain. Numerous researches have consistently reported that tau levels in the cerebrospinal fluid (CSF) are prominently increased in patients with AD compared to controls (84). Therefore, CSF, a method of accessing and evaluating brain metabolism, can be used to reflect the tau pathology and evaluate the effectiveness of therapeutics. Tau, serving as a core CSF biomarker for AD, including total tau (T-tau) and hyperphosphorylated tau (P-tau), has been successfully obtained by ELISA. T-tau is correlated with the intensity of neurodegeneration, while P-tau reflects the neurofibrillary pathological changes (85). Measuring these tau species could also contribute to the classification of AD from relevant differential diagnoses. P-tau181 and P-tau231 can be used to distinguish AD from control groups, FTD and dementia with Lewy bodies (86). As blood is more accessible than CSF, the tau proteins in plasma would be worthy of detection. There has been considerable data reporting that patients with MCI or early AD have higher plasma tau levels by using an immunomagnetic reduction assay (87). It is necessary to improve the specificity and enhance the accuracy for assays in order to make them useful for diagnosis and treatment of neurodegenerative disease.
Furthermore, as the tau imaging for neurofibrillary pathology has already been established, several potential imaging biomarkers have been developed in the past few years, including [18F]THK5117, [18F]THK5351, [18F]AV1451 (T807) and [11C]PBB3 (88). They bind to the tau in brain of neurodegenerative diseases and can track disease progression. In this stage, enhancing spatial resolution and specificity is the primary task.
Tau as a therapeutic target for neurodegenerative diseases
Microtubule stabilization
The dysfunction of tau protein, as mentioned above, is often associated with microtubule instability. Microtubule disruption has been described in several models, including transgenic mice overexpressing wild-type human tau (T44 model) or P301S human 4R1N tau (PS19 model) (89,90). The results of these studies have shown that maintaining microtubule stabilization could be an effective therapeutic strategy. The microtubule stabilizer epothilone D, which has favorable pharmacokinetic and pharmacodynamic profile, improves axonal transport and motor function and also increases the number of microtubules in transgenic mouse lines (91,92). Phase I clinical trials for mild AD have been carried out. Another microtubule stabilizer, davunetide (also referred to as NAP, a short peptide), was undergoing a phase II/III trial in PSP. However, it does not have any therapeutic benefit (93).
Inhibition of tau aggregation
Inhibiting tau aggregation is a promising therapeutic avenue. Phenothiazine methylene blue, which was considered as the first compound to prevent tau aggregation (94), demonstrated great efficiency in treating cognitive deficits in a phase II trial in mild to moderate AD. A derivative of methylene blue (LMTX) is undergoing phase III trials (95). In addition, evidence showed that several D-amino acid peptides relying intensively on the atomic structure of the tau-derived hexapeptide VQIVYK also inhibited tau aggregation in vitro (96).
Targeting post-translational modifications
The hyperphosphorylation of tau is believed to be an extremely important and necessary step in tau-mediated neurodegeneration, which can be achieved by inhibiting kinases or stimulating phosphatases, and thus it will be a therapeutic target for AD and other tauopathies.
Many tau kinases involving AD, GSK-3 and CDK5 are considered as the best targets for treatment (97-99). Tideglusib (NP-12), a specific GSK-3 inhibitor, has been tested in phase II clinical trials in AD and PSP. In AD, tideglusib produced no clinical benefit (100); whereas it reduced the progression of brain atrophy in PSP (101). Other kinases (CDK5, CK1 and MAPKs) are also considered as targets for the inhibition of tau phosphorylation. However, they have not entered clinical trials to date yet. PP2A, as the main tau phosphatase, has been applied as another therapeutic target. It consists of a catalytic (C), a scaffolding (A), and a regulatory (B) subunits. Folic acid, presenting at low concentrations in AD patients, contributes to increased homocysteine levels and promotes the methylation of PP2Ac, subsequently increasing the formation of active PP2A (102,103). Similarly, it was found that the anti-diabetic drug metformin and chronic low doses of sodium selenite also enhanced PP2A activity and weakened tau phosphorylation in mice (104,105).
Clearance of tau and tau pathology
Another attractive strategy is to enhance the clearance of tau and tau pathology. As we all know, the ubiquitin-proteasome and lysosomal pathways are considered as the major routes of tau degradation. Soluble tau mainly depends on the former. The carboxy-terminus of heat shock protein 70-interacting protein (CHIP), which is identified as the ubiquitin ligase, bonds with the phosphorylated tau, cooperates with ubiquitin and subsequently target tau to the proteasome (106). In the JNPL3 model (overexpressing P301L human 4R0N tau mice), the reduction of CHIP increased the accumulation of tau while reduced the CHIP levels in AD brains (107). Moreover, CHIP in collaboration with the Hsp90 chaperone complex also plays a critical role in tau degradation. Hsp90 levels were inversely proportional to tau oligomers and soluble tau (108). Thus, enhancing the ubiquitin-proteasome function could be considered as a valid therapeutic strategy for tauopathies.
In addition, aggregation of considerable tau could be degraded by the autophagy lysosomal system, including macroautophagy, microautophagy and chaperone mediated autophagy. Seemingly, using rapamycin [activates autophagy in rapamycin (mTOR)-dependent manner] or trehalose (activates autophagy in an mTOR-independent manner) serves as an effective approach to stimulate autophagy, reduce the amount of insoluble tau protein and suppress the tau-induced neurotoxicity (109-111).
Tau immunization
Immunotherapy is deemed as a promising therapeutic strategy for tauopathies. Based on the seven studies (five studies with active immunization against tau and two studies with passive immunization), both active and passive vaccinations have shown positive impacts on tau transgenic mice (112-118). Recently, Kfoury et al. reported that the tau aggregate propagation was blocked by an anti-tau monoclonal antibody in an in vitro system (119). Possibly, the antibodies also enter into neurons and decrease intracellular tau aggregates. Such antibodies might enter neurons via clathrin-mediated endocytosis following the binding to Fcγ receptors and prevent progressive neurodegeneration (120). The mode of activity of the tau-specific antibodies is still unknown.
Anti-inflammatory treatments
As known to all, inflammation significantly contributes to AD pathogenesis and also plays an important role in other tauopathies (121,122). Thus, treatments with anti-inflammatory drugs could also be an effective therapeutic target for tauopathies. Transgenic mice deprived of the microglial-specific fractalkine receptor CX3CR1, which exerts an anti-inflammatory impact on microglia, showed aggravating tau pathologies (123). Consistent with this result, experiments with overexpression fractalkine receptor null mice suggested that fractalkine signaling reduced the amount of tau pathologies (124). Several clinical trials targeting inflammation have been conducted, but the results are unclear.
Down-regulation of tau
Although which entities carry out neurotoxic functions in tauopathies is still unclear, studies concerning tau-knockout mice have demonstrated that tau levels are important for tau-mediated neurodegeneration. As an attractive treatment approach, down-regulation of tau might be achieved through antisense oligonucleotides, microRNAs (miRNAs), small interfering RNAs (siRNAs) or other transcription inhibitors.
Conclusions
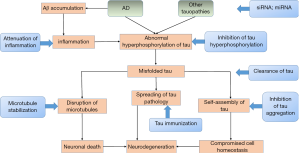
Hyperphosphorylated, aggregated tau is a central neuropathological characteristic of AD and many human neurodegenerative diseases. Since the discovery of mutations in the MAPT gene, considerable reports have been published on the role of tau in recent years. Depending on the development of various tau transgenic animal models, we have made substantial progress in tau-dependent pathologies, including the propagation of tau pathology from neurons to other cells and the interplay between Aβ and tau. Depending on those pathologies and tau-related biomarkers, new therapeutic approaches have been obtained (Figure 3). Nevertheless, the aetiological factors that initiate neurodegeneration and the precise mechanisms underlying tau-mediated neurotoxicity still need to be elucidated. We sincerely hope that further efforts could be made to clarify the role of tau in neurodegenerative diseases and advance more potential drugs into clinical trials.
Acknowledgements
This work was supported by grants from the Shandong Provincial Outstanding Medical Academic Professional Program, Qingdao Key Health Discipline Development Fund, Qingdao Outstanding Health Professional Development Fund, and Shan dong Provincial Collaborative Innovation Center for Neurodegenerative Disorders.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yu JT, Tan L, Hardy J. Apolipoprotein E in Alzheimer's disease: an update. Annu Rev Neurosci 2014;37:79-100. [Crossref] [PubMed]
- Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998;393:702-5. [Crossref] [PubMed]
- Morris M, Maeda S, Vossel K, et al. The many faces of tau. Neuron 2011;70:410-26. [Crossref] [PubMed]
- Goedert M, Spillantini MG, Jakes R, et al. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 1989;3:519-26. [Crossref] [PubMed]
- Goedert M, Spillantini MG, Potier MC, et al. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J 1989;8:393-9. [PubMed]
- Dixit R, Ross JL, Goldman YE, et al. Differential regulation of dynein and kinesin motor proteins by tau. Science 2008;319:1086-9. [Crossref] [PubMed]
- Mandell JW, Banker GA. A spatial gradient of tau protein phosphorylation in nascent axons. J Neurosci 1996;16:5727-40. [Crossref] [PubMed]
- Violet M, Delattre L, Tardivel M, et al. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front Cell Neurosci 2014;8:84. [Crossref] [PubMed]
- Sultan A, Nesslany F, Violet M, et al. Nuclear tau, a key player in neuronal DNA protection. J Biol Chem 2011;286:4566-75. [Crossref] [PubMed]
- Gheyara AL, Ponnusamy R, Djukic B, et al. Tau reduction prevents disease in a mouse model of Dravet syndrome. Ann Neurol 2014;76:443-56. [Crossref] [PubMed]
- Holth JK, Bomben VC, Reed JG, et al. Tau loss attenuates neuronal network hyperexcitability in mouse and Drosophila genetic models of epilepsy. J Neurosci 2013;33:1651-9. [Crossref] [PubMed]
- Leroy K, Ando K, Laporte V, et al. Lack of tau proteins rescues neuronal cell death and decreases amyloidogenic processing of APP in APP/PS1 mice. Am J Pathol 2012;181:1928-40. [Crossref] [PubMed]
- DeVos SL, Goncharoff DK, Chen G, et al. Antisense reduction of tau in adult mice protects against seizures. J Neurosci 2013;33:12887-97. [Crossref] [PubMed]
- Hong XP, Peng CX, Wei W, et al. Essential role of tau phosphorylation in adult hippocampal neurogenesis. Hippocampus 2010;20:1339-49. [Crossref] [PubMed]
- Fuster-Matanzo A, de Barreda EG, Dawson HN, et al. Function of tau protein in adult newborn neurons. FEBS Lett 2009;583:3063-8. [Crossref] [PubMed]
- Lei P, Ayton S, Finkelstein DI, et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med 2012;18:291-5. [Crossref] [PubMed]
- Kimura T, Whitcomb DJ, Jo J, et al. Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philos Trans R Soc Lond B Biol Sci 2013;369:20130144. [Crossref] [PubMed]
- Ahmed T, Van der Jeugd A, Blum D, et al. Cognition and hippocampal synaptic plasticity in mice with a homozygous tau deletion. Neurobiol Aging 2014;35:2474-8. [Crossref] [PubMed]
- Scheltens P, Blennow K, Breteler MM, et al. Alzheimer's disease. Lancet 2016;388:505-17. [Crossref] [PubMed]
- Jeganathan S, von Bergen M, Brutlach H, et al. Global hairpin folding of tau in solution. Biochemistry 2006;45:2283-93. [Crossref] [PubMed]
- Alonso A, Zaidi T, Novak M, et al. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A 2001;98:6923-8. [Crossref] [PubMed]
- Yuzwa SA, Cheung AH, Okon M, et al. O-GlcNAc modification of tau directly inhibits its aggregation without perturbing the conformational properties of tau monomers. J Mol Biol 2014;426:1736-52. [Crossref] [PubMed]
- Watanabe A, Hong WK, Dohmae N, et al. Molecular aging of tau: disulfide-independent aggregation and non-enzymatic degradation in vitro and in vivo. J Neurochem 2004;90:1302-11. [Crossref] [PubMed]
- Funk KE, Thomas SN, Schafer KN, et al. Lysine methylation is an endogenous post-translational modification of tau protein in human brain and a modulator of aggregation propensity. Biochem J 2014;462:77-88. [Crossref] [PubMed]
- Chung SH. Aberrant phosphorylation in the pathogenesis of Alzheimer's disease. BMB Rep 2009;42:467-74. [Crossref] [PubMed]
- Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med 2009;15:112-9. [Crossref] [PubMed]
- Liu F, Grundke-Iqbal I, Iqbal K, et al. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci 2005;22:1942-50. [Crossref] [PubMed]
- Iqbal K, Alonso Adel C, Chen S, et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta 2005;1739:198-210. [Crossref] [PubMed]
- Gong CX, Singh TJ, Grundke-Iqbal I, et al. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem 1993;61:921-7. [Crossref] [PubMed]
- Rahman A, Grundke-Iqbal I, Iqbal K. Phosphothreonine-212 of Alzheimer abnormally hyperphosphorylated tau is a preferred substrate of protein phosphatase-1. Neurochem Res 2005;30:277-87. [Crossref] [PubMed]
- Hoover BR, Reed MN, Su J, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 2010;68:1067-81. [Crossref] [PubMed]
- Thies E, Mandelkow EM. Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/Par-1. J Neurosci 2007;27:2896-907. [Crossref] [PubMed]
- Zempel H, Thies E, Mandelkow E, et al. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci 2010;30:11938-50. [Crossref] [PubMed]
- Guillozet-Bongaarts AL, Cahill ME, Cryns VL, et al. Pseudophosphorylation of tau at serine 422 inhibits caspase cleavage: in vitro evidence and implications for tangle formation in vivo. J Neurochem 2006;97:1005-14. [Crossref] [PubMed]
- Dickey CA, Kamal A, Lundgren K, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest 2007;117:648-58. [Crossref] [PubMed]
- Ittner LM, Ke YD, Gotz J. Phosphorylated Tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer disease. J Biol Chem 2009;284:20909-16. [Crossref] [PubMed]
- Min SW, Cho SH, Zhou Y, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 2010;67:953-66. [Crossref] [PubMed]
- Cohen TJ, Guo JL, Hurtado DE, et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun 2011;2:252. [Crossref] [PubMed]
- Irwin DJ, Cohen TJ, Grossman M, et al. Acetylated tau, a novel pathological signature in Alzheimer's disease and other tauopathies. Brain 2012;135:807-18. [Crossref] [PubMed]
- Wang JZ, Grundke-Iqbal I, Iqbal K. Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification in Alzheimer's disease. Nat Med 1996;2:871-5. [Crossref] [PubMed]
- Liu F, Iqbal K, Grundke-Iqbal I, et al. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer's disease. Proc Natl Acad Sci U S A 2004;101:10804-9. [Crossref] [PubMed]
- Reynolds MR, Reyes JF, Fu Y, et al. Tau nitration occurs at tyrosine 29 in the fibrillar lesions of Alzheimer's disease and other tauopathies. J Neurosci 2006;26:10636-45. [Crossref] [PubMed]
- Mori H, Kondo J, Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science 1987;235:1641-4. [Crossref] [PubMed]
- Cripps D, Thomas SN, Jeng Y, et al. Alzheimer disease-specific conformation of hyperphosphorylated paired helical filament-Tau is polyubiquitinated through Lys-48, Lys-11, and Lys-6 ubiquitin conjugation. J Biol Chem 2006;281:10825-38. [Crossref] [PubMed]
- Dorval V, Fraser PE. Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and alpha-synuclein. J Biol Chem 2006;281:9919-24. [Crossref] [PubMed]
- Lu PJ, Wulf G, Zhou XZ, et al. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 1999;399:784-8. [Crossref] [PubMed]
- Nakamura K, Greenwood A, Binder L, et al. Proline isomer-specific antibodies reveal the early pathogenic tau conformation in Alzheimer's disease. Cell 2012;149:232-44. [Crossref] [PubMed]
- Wischik CM, Novak M, Thogersen HC, et al. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A 1988;85:4506-10. [Crossref] [PubMed]
- Gamblin TC, Chen F, Zambrano A, et al. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci U S A 2003;100:10032-7. [Crossref] [PubMed]
- Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci 2016;17:5-21. [Crossref] [PubMed]
- Berger Z, Roder H, Hanna A, et al. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci 2007;27:3650-62. [Crossref] [PubMed]
- Flach K, Hilbrich I, Schiffmann A, et al. Tau oligomers impair artificial membrane integrity and cellular viability. J Biol Chem 2012;287:43223-33. [Crossref] [PubMed]
- Tian H, Davidowitz E, Lopez P, et al. Trimeric tau is toxic to human neuronal cells at low nanomolar concentrations. Int J Cell Biol 2013;2013:260787. [PubMed]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239-59. [Crossref] [PubMed]
- Takeda S, Wegmann S, Cho H, et al. Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer's disease brain. Nat Commun 2015;6:8490. [Crossref] [PubMed]
- Liu L, Drouet V, Wu JW, et al. Trans-synaptic spread of tau pathology in vivo. PLoS One 2012;7:e31302. [Crossref] [PubMed]
- de Calignon A, Polydoro M, Suarez-Calvet M, et al. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron 2012;73:685-97. [Crossref] [PubMed]
- Khan UA, Liu L, Provenzano FA, et al. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer's disease. Nat Neurosci 2014;17:304-11. [Crossref] [PubMed]
- Sanders DW, Kaufman SK, DeVos SL, et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 2014;82:1271-88. [Crossref] [PubMed]
- De Felice FG, Wu D, Lambert MP, et al. Alzheimer's disease-type neuronal tau hyperphosphorylation induced by A beta oligomers. Neurobiol Aging 2008;29:1334-47. [Crossref] [PubMed]
- Roberson ED, Scearce-Levie K, Palop JJ, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science 2007;316:750-4. [Crossref] [PubMed]
- Ittner LM, Ke YD, Delerue F, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell 2010;142:387-97. [Crossref] [PubMed]
- Roberson ED, Halabisky B, Yoo JW, et al. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. J Neurosci 2011;31:700-11. [Crossref] [PubMed]
- Hasegawa M, Smith MJ, Goedert M. Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett 1998;437:207-10. [Crossref] [PubMed]
- Hong M, Zhukareva V, Vogelsberg-Ragaglia V, et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 1998;282:1914-7. [Crossref] [PubMed]
- Rossi G, Tagliavini F. Frontotemporal lobar degeneration: old knowledge and new insight into the pathogenetic mechanisms of tau mutations. Front Aging Neurosci 2015;7:192. [Crossref] [PubMed]
- Alonso Adel C, Mederlyova A, Novak M, et al. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem 2004;279:34873-81. [Crossref] [PubMed]
- Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord 2002;16:203-12. [Crossref] [PubMed]
- Constantinidis J, Richard J, Tissot R. Pick's disease. Histological and clinical correlations. Eur Neurol 1974;11:208-17. [Crossref] [PubMed]
- Lang AE, Bergeron C, Pollanen MS, et al. Parietal Pick's disease mimicking cortical-basal ganglionic degeneration. Neurology 1994;44:1436-40. [Crossref] [PubMed]
- Graff-Radford NR, Damasio AR, Hyman BT, et al. Progressive aphasia in a patient with Pick's disease: a neuropsychological, radiologic, and anatomic study. Neurology 1990;40:620-6. [Crossref] [PubMed]
- Dickson DW. Neuropathology of Pick's disease. Neurology 2001;56:S16-20. [Crossref] [PubMed]
- Bronner IF, ter Meulen BC, Azmani A, et al. Hereditary Pick's disease with the G272V tau mutation shows predominant three-repeat tau pathology. Brain 2005;128:2645-53. [Crossref] [PubMed]
- Rockenstein E, Overk CR, Ubhi K, et al. A novel triple repeat mutant tau transgenic model that mimics aspects of pick's disease and fronto-temporal tauopathies. PLoS One 2015;10:e0121570. [Crossref] [PubMed]
- Tacik P, DeTure M, Hinkle KM, et al. A Novel Tau Mutation in Exon 12, p.Q336H, Causes Hereditary Pick Disease. J Neuropathol Exp Neurol 2015;74:1042-52. [Crossref] [PubMed]
- Yamada T, McGeer PL, McGeer EG. Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett 1992;135:99-102. [Crossref] [PubMed]
- Feany MB, Dickson DW. Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 1995;146:1388-96. [PubMed]
- Komori T, Arai N, Oda M, et al. Astrocytic plaques and tufts of abnormal fibers do not coexist in corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 1998;96:401-8. [Crossref] [PubMed]
- Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 1999;246 Suppl 2:II6-15. [Crossref] [PubMed]
- Dickson DW, Ahmed Z, Algom AA, et al. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 2010;23:394-400. [Crossref] [PubMed]
- Pittman AM, Myers AJ, Abou-Sleiman P, et al. Linkage disequilibrium fine mapping and haplotype association analysis of the tau gene in progressive supranuclear palsy and corticobasal degeneration. J Med Genet 2005;42:837-46. [Crossref] [PubMed]
- Stanford PM, Halliday GM, Brooks WS, et al. Progressive supranuclear palsy pathology caused by a novel silent mutation in exon 10 of the tau gene: expansion of the disease phenotype caused by tau gene mutations. Brain 2000;123:880-93. [Crossref] [PubMed]
- Bugiani O, Murrell JR, Giaccone G, et al. Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol 1999;58:667-77. [Crossref] [PubMed]
- Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer's disease. NeuroRx 2004;1:213-25. [Crossref] [PubMed]
- Blennow K, Hampel H, Weiner M, et al. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 2010;6:131-44. [Crossref] [PubMed]
- Lu H, Zhu XC, Jiang T, et al. Body fluid biomarkers in Alzheimer's disease. Ann Transl Med 2015;3:70. [PubMed]
- Chiu MJ, Chen YF, Chen TF, et al. Plasma tau as a window to the brain-negative associations with brain volume and memory function in mild cognitive impairment and early Alzheimer's disease. Hum Brain Mapp 2014;35:3132-42. [Crossref] [PubMed]
- Dani M, Edison P, Brooks DJ. Imaging biomarkers in tauopathies. Parkinsonism Relat Disord 2016;22 Suppl 1:S26-8. [Crossref] [PubMed]
- Zhang B, Maiti A, Shively S, et al. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc Natl Acad Sci U S A 2005;102:227-31. [Crossref] [PubMed]
- Yoshiyama Y, Higuchi M, Zhang B, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007;53:337-51. [Crossref] [PubMed]
- Brunden KR, Zhang B, Carroll J, et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J Neurosci 2010;30:13861-6. [Crossref] [PubMed]
- Zhang B, Carroll J, Trojanowski JQ, et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci 2012;32:3601-11. [Crossref] [PubMed]
- Boxer AL, Lang AE, Grossman M, et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol 2014;13:676-85. [Crossref] [PubMed]
- Wischik CM, Edwards PC, Lai RY, et al. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci U S A 1996;93:11213-8. [Crossref] [PubMed]
- Wischik CM, Staff RT, Wischik DJ, et al. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer's disease. J Alzheimers Dis 2015;44:705-20. [PubMed]
- Sievers SA, Karanicolas J, Chang HW, et al. Structure-based design of non-natural amino-acid inhibitors of amyloid fibril formation. Nature 2011;475:96-100. [Crossref] [PubMed]
- Pei JJ, Grundke-Iqbal I, Iqbal K, et al. Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer's disease neurofibrillary degeneration. Brain Res 1998;797:267-77. [Crossref] [PubMed]
- Pei JJ, Braak E, Braak H, et al. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol 1999;58:1010-9. [Crossref] [PubMed]
- Kremer A, Louis JV, Jaworski T, et al. GSK3 and Alzheimer's Disease: Facts and Fiction. Front Mol Neurosci 2011;4:17. [Crossref] [PubMed]
- Lovestone S, Boada M, Dubois B, et al. A phase II trial of tideglusib in Alzheimer's disease. J Alzheimers Dis 2015;45:75-88. [PubMed]
- Hoglinger GU, Huppertz HJ, Wagenpfeil S, et al. Tideglusib reduces progression of brain atrophy in progressive supranuclear palsy in a randomized trial. Mov Disord 2014;29:479-87. [Crossref] [PubMed]
- Sontag JM, Nunbhakdi-Craig V, Montgomery L, et al. Folate deficiency induces in vitro and mouse brain region-specific downregulation of leucine carboxyl methyltransferase-1 and protein phosphatase 2A B(alpha) subunit expression that correlate with enhanced tau phosphorylation. J Neurosci 2008;28:11477-87. [Crossref] [PubMed]
- Wu J, Tolstykh T, Lee J, et al. Carboxyl methylation of the phosphoprotein phosphatase 2A catalytic subunit promotes its functional association with regulatory subunits in vivo. EMBO J 2000;19:5672-81. [Crossref] [PubMed]
- van Eersel J, Ke YD, Liu X, et al. Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer's disease models. Proc Natl Acad Sci U S A 2010;107:13888-93. [Crossref] [PubMed]
- Kickstein E, Krauss S, Thornhill P, et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci U S A 2010;107:21830-5. [Crossref] [PubMed]
- Petrucelli L, Dickson D, Kehoe K, et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet 2004;13:703-14. [Crossref] [PubMed]
- Sahara N, Murayama M, Mizoroki T, et al. In vivo evidence of CHIP up-regulation attenuating tau aggregation. J Neurochem 2005;94:1254-63. [Crossref] [PubMed]
- Sahara N, Maeda S, Yoshiike Y, et al. Molecular chaperone-mediated tau protein metabolism counteracts the formation of granular tau oligomers in human brain. J Neurosci Res 2007;85:3098-108. [Crossref] [PubMed]
- Kruger U, Wang Y, Kumar S, et al. Autophagic degradation of tau in primary neurons and its enhancement by trehalose. Neurobiol Aging 2012;33:2291-305. [Crossref] [PubMed]
- Ozcelik S, Fraser G, Castets P, et al. Rapamycin attenuates the progression of tau pathology in P301S tau transgenic mice. PLoS One 2013;8:e62459. [Crossref] [PubMed]
- Berger Z, Ravikumar B, Menzies FM, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet 2006;15:433-42. [Crossref] [PubMed]
- Asuni AA, Boutajangout A, Quartermain D, et al. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci 2007;27:9115-29. [Crossref] [PubMed]
- Boimel M, Grigoriadis N, Lourbopoulos A, et al. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp Neurol 2010;224:472-85. [Crossref] [PubMed]
- Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci 2010;30:16559-66. [Crossref] [PubMed]
- Boutajangout A, Ingadottir J, Davies P, et al. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem 2011;118:658-67. [Crossref] [PubMed]
- Chai X, Wu S, Murray TK, et al. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J Biol Chem 2011;286:34457-67. [Crossref] [PubMed]
- Bi M, Ittner A, Ke YD, et al. Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PLoS One 2011;6:e26860. [Crossref] [PubMed]
- Troquier L, Caillierez R, Burnouf S, et al. Targeting phospho-Ser422 by active Tau Immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr Alzheimer Res 2012;9:397-405. [Crossref] [PubMed]
- Kfoury N, Holmes BB, Jiang H, et al. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem 2012;287:19440-51. [Crossref] [PubMed]
- Kondo A, Shahpasand K, Mannix R, et al. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 2015;523:431-6. [Crossref] [PubMed]
- Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer's disease. Neurobiol Aging 2000;21:383-421. [Crossref] [PubMed]
- Tarkowski E, Andreasen N, Tarkowski A, et al. Intrathecal inflammation precedes development of Alzheimer's disease. J Neurol Neurosurg Psychiatry 2003;74:1200-5. [Crossref] [PubMed]
- Bhaskar K, Konerth M, Kokiko-Cochran ON, et al. Regulation of tau pathology by the microglial fractalkine receptor. Neuron 2010;68:19-31. [Crossref] [PubMed]
- Nash KR, Lee DC, Hunt JB Jr, et al. Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy. Neurobiol Aging 2013;34:1540-8. [Crossref] [PubMed]


