Association of lectin-like oxidized low density lipoprotein receptor 1 (OLR1) polymorphisms with late-onset Alzheimer disease in Han Chinese
Introduction
Alzheimer’s disease (AD) is a common cause for dementia among old people. It could cause progressive cognitive impairment, accumulation of amyloid plaques (AP) and intracellular neurofibrillary tangles (NFTs), and neuronal loss in brain (1,2). The pathogenesis of AD is very complex. A number of studies have suggested that the onset of AD was influenced by genetic risk factors (3). Presenilin 1 (PSEN1), presenilin 2 (PSEN2), and amyloid precursor protein (APP) gene mutations bear responsibility for most familial AD cases (4). The ε4 allele of apolipoprotein E (APOE) is widely recognized for the more common sporadic late-onset AD (LOAD). APOE has been identified as a major transporter of cholesterol both in the blood and central nervous system (5). Based on the role of APOE for the pathogenesis of AD and some other findings, we hypothesized that imbalanced cholesterol levels may influence the risk of AD, and statins and other cholesterol-modifying medications may be useful in AD’s treatment and prevention (6-16).
Chromosome 12p has been recognized as a region associated with AD by Genome-wide linkage analyses. It includes lipoprotein receptor-related protein 1 (LRP1), α-2-macrogobulin (A2M), as well as OLR1 (17). OLR1, a class E scavenger receptor, is a trans-membrane glycoprotein. It could mediate the uptake and internalization of oxidized low-density lipoprotein (oxLDL) (18). In vitro factors such as oxLDL, oxidative stress and inflammatory cytokines, and in vivo proatherogenic stimuli such as diabetes mellitus, hyperlipidaemia, as well as hypertension could induce its expression (19-21). Increased level of oxLDL induces endothelial cell activation, dysfunction, apoptosis and impaired vasorelaxation, thus contribute causally to atherosclerosis development and progression (22-28). Indeed, epidemiologic and clinical literature has consistently reported an association between atherosclerosis, vascular risk factors and AD (29,30). Therefore, it is possible that variations in ORL1 could lead to oxLDL being removed less efficiently, and this, with increased amyloid beta peptide (Aβ) might result in death of neurons (31-33).
MicroRNAs (miRNAs) are 19–24 nt single-stranded RNA molecules. It can down-regulate gene expression through binding to a complementary sequence in the 3'UTR of target genes, thus resulting in translational inhibition or mRNA cleavage and subsequent degradation (34). Clinical and research evidence showed that a number of miRNAs are dysregulated in AD patients and AD animal models (35-39). The single-nucleotide polymorphism rs1050283 (also known as +1073 C/T) located in the 3'UTR of OLR1 may influence its regulator miRNA binding and subsequent protein homeostasis. Several studies have explored the association between OLR1 +1073 C/T and AD. However, the results are inconsistent (17,40-43). Recently, a meta-analysis confirmed the association of OLR1+1073 C/T with a decreased risk of AD among Caucasians (44). Moreover, Papassotiropoulos et al. investigated a cluster of cholesterol-related genes and identified rs1050286 polymorphism in OLR1 conferring significant susceptibility for AD (45). Given the potential importance of OLR1 in the pathogenesis and development of AD, we performed a case-control study consisting of 984 patients with LOAD as compared to 1,354 age-matched healthy subjects among northern Han Chinese, by analyzing the potentially association of three functional SNPs (rs1050283, rs1050286, rs17808009) located in 3'UTR of OLR1 with LOAD in Han Chinese population.
Methods
Subjects
Totally, 984 sporadic LOAD patients and 1,354 healthy controls were enrolled for the study. They were matched for age and sex. The cases came from several hospitals in Shandong Province. All participants were northern Han Chinese. The diagnosis of AD was carried out with standard clinical evaluation in accordance with NINCDS-ADRDA criteria (46). No patients had severe CNS diseases or a family history of dementia. The controls were confirmed healthy and neurologically normal by medical history, general examinations, laboratory examinations and Mini Mental State Examination MMSE (score ≥28). They were enrolled from the Health Examination Center of the Qingdao Municipal Hospital. The present study was carried out with approval by the Institute Ethical Committee of Qingdao Municipal Hospital and with informed consent of all the participants or their representatives. The ID number of informed consent is 2009-05-06-003.
SNP selection and genotype
Candidate SNPs meet the following criterion: (I) SNPs of miRNA binding sites located in the 3'UTR region of OLR1; (II) SNPs from the public databases and literatures; (III) SNPs with a minor allele frequency (MAF) ≥0.05 in Han Chinese. Finally, three SNPs (rs1050283, rs1050286, rs17808009) were chosen and genotyped.
Standard procedures were used for extraction of human genomic DNA. Genotyping of OLR1 polymorphisms (rs1050286and rs17808009) and APOE polymorphisms (rs429358 and rs7412) were determined by a custom-by-design 2-×48-Plex SNPscanTM kit (Genesky Biotechnologies Inc., Shanghai, China).
The sequence of probes in SNP scan reaction and sequence for the PCR reaction are available from the corresponding author. The improved multiplex ligase detection reaction (iMLDR) method was used for detection of genotyping of rs1050283 in the OLR1 gene (Shanghai Genesky Bio-Tech Co, Ltd; www.geneskies.com). Primers for rs1050283 are as follows: forward primer: CTTGATTTCGGAATGGCCTCTG; reverse primer: CCTTTGCAGAAACTGGGGTTCC. Data analysis was performed via an ABI3130XL Sequencer and GeneMapper Software v4.1 (Applied Biosystems, USA).
Statistical analyses
Differences in the characteristics between two groups were assessed with the χ2 test or Student t-test. Hardy-Weinberg equilibrium was examined with the χ2 test. The χ2 test was used to compare distributions of genotype and allele, and was also used to test differences between two groups stratified by APOEε4 status. Differences in allele and genotype distribution between two groups were analyzed with logistic regression adjusted for gender, age and APOEε4 status in several genetic models. The models were defined as 0 (AA + Aa) versus 1 (aa) for recessive, 2 (aa) versus 1 (Aa) versus 0 (AA) for additive, and 0 (AA) versus 1 (Aa + aa) for dominant, (A: major allele; a: minor allele). STPLAN4.5 software was used to estimate statistical power. SPSS 16.0 software was used for data analysis. P<0.05 were considered to have statistical significance.
Results
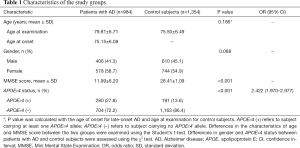
Demographic and clinical characteristics of LOAD and healthy controls are shown in Table 1. The two groups were well-matched with regard to age (P=0.186) and gender (P=0.068). LOAD subjects had much lower MMSE scores than controls (P<0.001). Bearing of the APOE ε4 allele was related to an elevated risk for LOAD as expected (OR =2.422, 95% CI: 1.970–2.977, P<0.001).
Full table
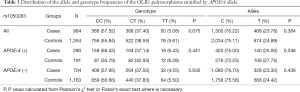
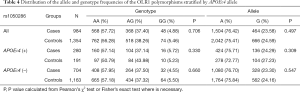
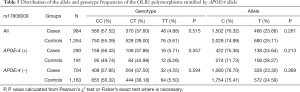
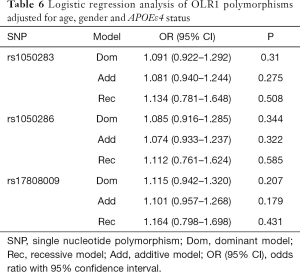
Table 2 summarized the details of the SNPs detected in our study. The distributions of rs1050283, rs1050286 and rs17808009 in both groups were all in HWE (P>0.05). The genotypes and allele frequencies of rs1050283, rs1050286 and rs17808009 in LOAD patients and controls in the total sample and after stratification for APOEε4 allele are presented in Tables 3-5. Though the frequencies of the minor alleles of all SNPs within OLR1 were reduced in patients compared with control subjects, no statistically significant association was observed for genotypic (P=0.675, P=0.706, P=0.515 for rs1050283, rs1050286 and rs17808009, respectively) and allelic(OR =0.942, 95% CI: 0.822–1.078, P=0.384, OR =0.954, 95% CI: 0.833–1.093, P=0.497, OR =0.925, 95% CI: 0.808–1.059, P=0.261 for rs1050283, rs1050286 and rs17808009, respectively) frequencies between LOAD patients and controls. Moreover, no differences were found in the genotypic or allelic distributions between the two groups even after stratification by APOEε4 status (Tables 3-5). Furthermore, no significant difference was detected between LOAD patients and healthy controls by the multivariate logistic regression with adjustment for non-genetic and the bearing of at least one APOEε4 allele (Table 6).

Full table
Full table
Full table
Full table
Full table
Discussion
As an endothelial receptor for oxLDL, OLR1 has been suggested to regulate lipid metabolism, and thus being an intriguing candidate gene for AD susceptibility. We sought to determine whether SNPs in OLR1 are involved with AD. In our research, we investigated three SNPs (rs1050283, rs1050286, rs17808009) for miRNA binding sites of 3'UTR in OLR1. Notably, we describe a novel polymorphism rs17808009. However, we are unable to replicate the findings in a case-control sample with adequate power to detect the risk ratio observed in previous studies. No significant differences in the genotypic or allelic distributions of these three SNPs (rs1050283, rs1050286, rs17808009) between LOAD subjects and controls was found in a Han Chinese population, even after statistical adjustment for non-genetic and APOEε4 status and stratification for APOEε4 status.
Previous human genetic association studies of the OLR1 have yielded conflicting results (17,33,40,41,43,47,48). Luedecking-Zimmer et al. (17) found that in APOEε4 bearers homozygosity of the T allele of the ORL1 rs1050283 polymorphism had an elevated risk for LOAD, while in non-ε4 carriers, homozygote of the T allele was over-represented in the controls. On the contrary, Lambert et al. (40) found that the expression of OLR1 was decreased in AD cases carrying the CC and CT genotypes compared with controls with the same genotypes (OR =1.56, 95% CI: 1.19–2.04, P<0.001), which indicated C allele to be a risk factor for AD. This was supported by two other studies (41,43). Recently, a meta-analysis including 2,419 cases and 2,381 controls from five studies demonstrated a significantly lower AD risk in the recessive model (TT vs. TC + CC: OR =0.79, 95% CI: 0.65–0.96), however, they failed to conduct further study to confirm the influence of APOEε4 on the association between rs1050283 polymorphism and AD (44). The results of an independent replication study by Pritchard et al. provided support to our research (48), they performed an association study of rs1050283 in a UK population of 356 LOAD patients and 358 healthy controls, and failed to find any association between rs1050283 and AD, even after stratification by APOEε4 status, onset age and haplotype distributions. In addition, Papassotiropoulos et al. (45) investigated a cluster of cholesterol-related genes and identified rs1050286 polymorphism in OLR1 conferring significant susceptibility to AD, which was inconsistent with our findings. Moreover, there is another SNP site known as +1071 T/A (not included in our study) within the OLR13'UTR, being tested by three groups (40,41,48). However, no relationship was found between the +1071 T/A polymorphism and AD onset risk.
A number of studies have explored the reasons for the frequent failure of candidate gene studies for replication in other cohorts (49-52). Our study failed to detect any association does not mean invalidation of previous findings. There are some explanations. Firstly, the difference between our study and previous research might be due to genetic variability in various ethnic populations, including differences in minor allele and MAF, as well as the complexity of the potential genetic structure (53,54). In Caucasians C allele was the minor allele of the rs1050283 polymorphism with a MAF of 0.482, while in our cohort T was the minor allele with a MAF of 0.238. Our information was similar to those from NCBI database (MAF: T=0.183). Candidate gene studies might differ in the study population and in the definition of the phenotype (55). Secondly, it is possible that our study is under-powered to detect small size effect in the Han Chinese population (56). In fact, our sample had a power of more than 90% to detect these variants with modest risk (MAF =0.25 and OR of ~1.5) at a significant level (alpha) of 0.05. However, we cannot deny that our results could be under-powered in case of weak effects. Thirdly, AD is not only a genetic disease, but also involves environmental components. Therefore, the effects of some SNPs detected by GWAS may vary in different populations owing to some undiscovered interactions between gene and environment (57). Moreover, variations in the clinical characteristics of the study cohort, as well as experimental and statistical methods could have caused statistical bias (58,59). More analyses with independent follow up are required to evaluate these possibilities.
To conclude, we failed to find any significant differences between SNPs (rs1050283, rs1050286, rs17808009) and LOAD in the Han Chinese population. As far as we know, this is the first study aimed at exploring the possible effects of the SNPs (rs1050283, rs1050286, rs17808009) in OLR1 to LOAD in non-Caucasians. Further and larger studies in Han Chinese and other ethnic groups are warranted to assess our results.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (81471309, 81371406, 81571245, and 81501103), the Shandong Provincial Outstanding Medical Academic Professional Program, Qingdao Key Health Discipline Development Fund, Qingdao Outstanding Health Professional Development Fund, and Shandong Provincial Collaborative Innovation Center for Neurodegenerative Disorders.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The present study was carried out with approval by the Institute Ethical Committee of Qingdao Municipal Hospital and with informed consent of all the participants or their representatives. The ID number of informed consent is 2009-05-06-003.
References
- Jiang T, Yu JT, Tian Y, et al. Epidemiology and etiology of Alzheimer's disease: from genetic to non-genetic factors. Curr Alzheimer Res 2013;10:852-67. [Crossref] [PubMed]
- Jiang T, Yu JT, Zhu XC, et al. Angiotensin-(1-7) induces cerebral ischaemic tolerance by promoting brain angiogenesis in a Mas/eNOS-dependent pathway. Br J Pharmacol 2014;171:4222-32. [Crossref] [PubMed]
- Wan Y, Wang G, Chen SD. Genetic predisposition to inflammation: a new risk factor of Alzheimer's disease. Neurosci Bull 2008;24:314-22. [Crossref] [PubMed]
- Bertram L, Tanzi RE. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci 2008;9:768-78. [Crossref] [PubMed]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 1988;240:622-30. [Crossref] [PubMed]
- Shobab LA, Hsiung GY, Feldman HH. Cholesterol in Alzheimer's disease. Lancet Neurol 2005;4:841-52. [Crossref] [PubMed]
- Wolozin B. Cholesterol and the biology of Alzheimer's disease. Neuron 2004;41:7-10. [Crossref] [PubMed]
- Wolozin B, Wang SW, Li NC, et al. Simvastatin is associated with a reduced incidence of dementia and Parkinson's disease. BMC Med 2007;5:20. [Crossref] [PubMed]
- Hartmann T. Cholesterol, A beta and Alzheimer's disease. Trends Neurosci 2001;24:S45-8. [Crossref] [PubMed]
- Bradley-Whitman MA, Lovell MA. Biomarkers of lipid peroxidation in Alzheimer disease (AD): an update. Arch Toxicol 2015;89:1035-44. [Crossref] [PubMed]
- Haag MD, Hofman A, Koudstaal PJ, et al. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry 2009;80:13-7. [Crossref] [PubMed]
- Li G, Shofer JB, Rhew IC, et al. Age-varying association between statin use and incident Alzheimer's disease. J Am Geriatr Soc 2010;58:1311-7. [Crossref] [PubMed]
- Bettermann K, Arnold AM, Williamson J, et al. Statins, risk of dementia, and cognitive function: secondary analysis of the ginkgo evaluation of memory study. J Stroke Cerebrovasc Dis 2012;21:436-44. [Crossref] [PubMed]
- Sparks DL, Kryscio RJ, Sabbagh MN, et al. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res 2008;5:416-21. [Crossref] [PubMed]
- Swiger KJ, Manalac RJ, Blumenthal RS, et al. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc 2013;88:1213-21. [Crossref] [PubMed]
- Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med 2013;159:688-97. [Crossref] [PubMed]
- Luedecking-Zimmer E, DeKosky ST, Chen Q, et al. Investigation of oxidized LDL-receptor 1 (OLR1) as the candidate gene for Alzheimer's disease on chromosome 12. Hum Genet 2002;111:443-51. [Crossref] [PubMed]
- Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997;386:73-7. [Crossref] [PubMed]
- Kume N, Murase T, Moriwaki H, et al. Inducible expression of lectin-like oxidized LDL receptor-1 in vascular endothelial cells. Circ Res 1998;83:322-7. [Crossref] [PubMed]
- Aoyama T, Sawamura T, Furutani Y, et al. Structure and chromosomal assignment of the human lectin-like oxidized low-density-lipoprotein receptor-1 (LOX-1) gene. Biochem J 1999;339:177-84. [Crossref] [PubMed]
- Minami M, Kume N, Kataoka H, et al. Transforming growth factor-beta(1) increases the expression of lectin-like oxidized low-density lipoprotein receptor-1. Biochem Biophys Res Commun 2000;272:357-61. [Crossref] [PubMed]
- Parthasarathy S, Raghavamenon A, Garelnabi MO, et al. Oxidized low-density lipoprotein. Methods Mol Biol 2010;610:403-17. [Crossref] [PubMed]
- Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic Biol Med 2000;28:1815-26. [Crossref] [PubMed]
- Parthasarathy S, Santanam N, Ramachandran S, et al. Oxidants and antioxidants in atherogenesis. An appraisal. J Lipid Res 1999;40:2143-57. [PubMed]
- Geekiyanage H, Chan C. MicroRNA-137/181c regulates serine palmitoyltransferase and in turn amyloid beta, novel targets in sporadic Alzheimer's disease. J Neurosci 2011;31:14820-30. [Crossref] [PubMed]
- Jayadev S, Case A, Alajajian B, et al. Presenilin 2 influences miR146 level and activity in microglia. J Neurochem 2013;127:592-9. [Crossref] [PubMed]
- Dickson JR, Kruse C, Montagna DR, et al. Alternative polyadenylation and miR-34 family members regulate tau expression. J Neurochem 2013;127:739-49. [Crossref] [PubMed]
- Absalon S, Kochanek DM, Raghavan V, et al. MiR-26b, upregulated in Alzheimer's disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons. J Neurosci 2013;33:14645-59. [Crossref] [PubMed]
- Guerreiro RJ, Gustafson DR, Hardy J. The genetic architecture of Alzheimer's disease: beyond APP, PSENs and APOE. Neurobiol Aging 2012;33:437-56. [Crossref] [PubMed]
- Rocchi A, Orsucci D, Tognoni G, et al. The role of vascular factors in late-onset sporadic Alzheimer's disease. Genetic and molecular aspects. Curr Alzheimer Res 2009;6:224-37. [Crossref] [PubMed]
- Keller JN, Hanni KB, Markesbery WR. Oxidized low-density lipoprotein induces neuronal death: implications for calcium, reactive oxygen species, and caspases. J Neurochem 1999;72:2601-9. [Crossref] [PubMed]
- Keller JN, Hanni KB, Kindy MS. Oxidized high-density lipoprotein induces neuron death. Exp Neurol 2000;161:621-30. [Crossref] [PubMed]
- Shi J, Tian J, Pritchard A, et al. A 3'-UTR polymorphism in the oxidized LDL receptor 1 gene increases Abeta40 load as cerebral amyloid angiopathy in Alzheimer's disease. Acta Neuropathol 2006;111:15-20. [Crossref] [PubMed]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281-97. [Crossref] [PubMed]
- Tan L, Yu JT, Tan L. Causes and Consequences of MicroRNA Dysregulation in Neurodegenerative Diseases. Mol Neurobiol 2015;51:1249-62. [Crossref] [PubMed]
- Hebert SS, De Strooper B. Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci 2009;32:199-206. [Crossref] [PubMed]
- Patel N, Hoang D, Miller N, et al. MicroRNAs can regulate human APP levels. Mol Neurodegener 2008;3:10. [Crossref] [PubMed]
- Hebert SS, Horre K, Nicolai L, et al. MicroRNA regulation of Alzheimer's Amyloid precursor protein expression. Neurobiol Dis 2009;33:422-8. [Crossref] [PubMed]
- Wang WX, Rajeev BW, Stromberg AJ, et al. The expression of microRNA miR-107 decreases early in Alzheimer's disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci 2008;28:1213-23. [Crossref] [PubMed]
- Lambert JC, Luedecking-Zimmer E, Merrot S, et al. Association of 3'-UTR polymorphisms of the oxidised LDL receptor 1 (OLR1) gene with Alzheimer's disease. J Med Genet 2003;40:424-30. [Crossref] [PubMed]
- D’Introno A, Solfrizzi V, Colacicco AM, et al. Polymorphisms in the Oxidized Low-Density Lipoprotein Receptor-1 Gene and Risk of Alzheimer’s Disease. J Gerontol A Biol Sci Med Sci 2005;60:280-4. [Crossref] [PubMed]
- Colacicco AM, Solfrizzi V, D'Introno A, et al. Alpha-2-macroglobulin gene, oxidized low-density lipoprotein receptor-1 locus, and sporadic Alzheimer's disease. Neurobiol Aging 2009;30:1518-20. [Crossref] [PubMed]
- Serpente M, Fenoglio C, Villa C, et al. Role of OLR1 and its regulating hsa-miR369-3p in Alzheimer's disease: genetics and expression analysis. J Alzheimers Dis 2011;26:787-93. [Crossref] [PubMed]
- Kong Y, Wu JB, Wang X, et al. Polymorphism of the OLR1 3'UTR potential microRNA binding site and risk of Alzheimer's disease: a meta-analysis. Genet Mol Res 2014;13:10162-72. [Crossref] [PubMed]
- Papassotiropoulos A, Wollmer MA, Tsolaki M, et al. A cluster of cholesterol-related genes confers susceptibility for Alzheimer's disease. J Clin Psychiatry 2005;66:940-7. [Crossref] [PubMed]
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939-44. [Crossref] [PubMed]
- Bertram L. No association between a previously reported OLR1 3' UTR polymorphism and Alzheimer's disease in a large family sample. Journal of Medical Genetics 2004;41:286-8. [Crossref] [PubMed]
- Pritchard A, St Clair D, Lemmon H, et al. No association between polymorphisms in the lectin-like oxidised low density lipoprotein receptor (ORL1) gene on chromosome 12 and Alzheimer's disease in a UK cohort. Neurosci Lett 2004;366:126-9. [Crossref] [PubMed]
- Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science 1996;273:1516-7. [Crossref] [PubMed]
- Boerwinkle E, Hixson JE, Hanis CL. Peeking Under the Peaks Following Up Genome-Wide Linkage Analyses. Circulation 2000;102:1877-78. [Crossref] [PubMed]
- Tabor HK, Risch NJ, Myers RM. Candidate-gene approaches for studying complex genetic traits: practical considerations. Genetics 2002;3:391-7. [PubMed]
- Botstein D, Risch N. Discovering genotypes underlying human phenotypes: past successes for mendelian disease, future approaches for complex disease. Nat Genet 2003;33 Suppl:228-37. [Crossref] [PubMed]
- Liu QY, Miao D, Yu JT, et al. Lack of association between rs597668 polymorphism near EXOC3L2 and late-onset Alzheimer's disease in Han Chinese. Neurosci Lett 2012;513:174-7. [Crossref] [PubMed]
- Zhou J, Li XM, Jiang T, et al. Lack of association between COMT Val158Met polymorphism and late-onset Alzheimer's disease in Han Chinese. Neurosci Lett 2013;554:162-6. [Crossref] [PubMed]
- Noble EP. The D2 dopamine receptor gene: a review of association studies in alcoholism and phenotypes. Alcohol 1998;16:33-45. [Crossref] [PubMed]
- Tan L, Yu JT, Zhang W, et al. Association of GWAS-linked loci with late-onset Alzheimer's disease in a northern Han Chinese population. Alzheimers Dement 2013;9:546-53. [Crossref] [PubMed]
- Pearson TA, Manolio TA. How to interpret a genome-wide association study. JAMA 2008;299:1335-44. [Crossref] [PubMed]
- Bertram L, Tanzi RE. Alzheimer's disease: one disorder, too many genes? Hum Mol Genet 2004;13:R135-41. [Crossref] [PubMed]
- Yu JT, Song JH, Ma T, et al. Genetic association of PICALM polymorphisms with Alzheimer's disease in Han Chinese. J Neurol Sci 2011;300:78-80. [Crossref] [PubMed]


