The practical benefit of Phadiatop test as the first-line in vitro allergen-specific immunoglobulin E (sIgE) screening of aeroallergens among Chinese asthmatics: a validation study
Introduction
Along with advances in biomedical sciences, the mainstream techniques for diagnosis of allergy have always been evolving, from the skin scratch test described by Blackley in 1873 (1), the enzyme-linked immunosorbent assay (ELISA) first published by Engvail and Perlmann in 1971 (2), radiation allergen sorbent test (RAST) developed in 1974 (3), to the ImmunoCAP assay (formerly the Pharmacia CAP) introduced in 1989 and used as the gold standard for in vitro serum tests so far (4,5). The ImmunoCAP assay, using cellulose as a solid phase covalently coupled to the allergen of interest and mounted in a cap-like structure, is now widely recognized and available for detection of allergen-specific immunoglobulin E (sIgE) in clinical settings (6). However, efficiency of an allergen sIgE detection strategy does not depend merely on the accuracy of an individual assay but also on the cost-effective size of allergen test panel (the number of allergens needed to detect), given that thousands of allergens worldwide or hundreds in a vast region could not be massively tested for an individual suspected to have atopy (7). This particularly applies to China, where in vitro allergen screening was introduced late, and data on local profiles of allergens remain limited. Furthermore, in this heavily populated country and others alike, geographical areas spanning multiple climate zones give rise to a complexity and diversity of allergen sensitization across different regions. To Chinese allergists, a dire need for cost-effective strategy of allergen detection is inarguable.
Unlike ImmunoCAP assay for individual allergens or multi-allergen mixes (usually of a same biological species), Phadiatop test is a commercially available variant of ImmunoCAP assay that uses a solid-phase coupled with a balanced mixture of common aeroallergens covering multiple species including fungi, pollens, insects and dust mites (8). This technique simultaneously screens patient sensitization to common aeroallergens but not to food, chemicals or other rare materials. Nevertheless, as far as inhalant allergens are concerned, we speculated that an initial screening with Phadiatop effectively ruling out majority of non-atopy would translate into a large saving of medical cost; then aided with local epidemiological data, the range of subsequent test panel in atopic subjects may be greatly narrowed. If so, Phadiatop would be a cost-effective approach for allergy diagnosis in countries with a large population and developing economy. To this regard, evidence on the clinical value of Phadiatop screening in China, especially in terms of its diagnostic efficiency, ought to be available, but unfortunately, is currently lacking.
Here we present our investigation about Phadiatop testing of serum samples validated by previous findings of ImmunoCAP assays in a Chinese cohort, with preliminary reflection on the implications of Phadiatop screening in developing countries with limited medical budgets.
Methods
Ethics statement
This study was approved by the Ethics Committee of First Affiliated Hospital of Guangzhou Medical University, with approval number: GYFYY-2016-73. The use of human serum samples was in accordance with the legislation in China and the wishes of donors, their legal guardians or next of kin, where applicable, who had offered written informed consent to using the serum samples for future unspecified research purposes.
Serum samples and study design
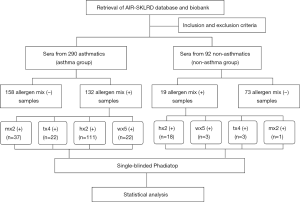
This was a validation study using serum samples registered between January 2015 and March 2017 in the Allergy Information Repository of State Key Laboratory of Respiratory Disease (AIR-SKLRD) located in southern China. The AIR-SKLRD is a large archive of medical records and serum biobank of subjects referred for allergen tests (9-11) with informed consent. We retrieved the AIR-SKLRD database for serum samples from consecutive asthma patients with during the study period, who had been tested simultaneously for four common aeroallergen mixes using an ImmunoCAP 1000 system (ThermoFisher Scientific, Uppsala, Sweden): house dusts mix (hx2), molds and yeasts mix (mx2), tree pollen mix (tx4) and weed pollen mix (wx5). In addition, we retrieved in the AIR-SKLRD database for serum samples from a contemporary cohort of health checkup subjects, who volunteered to undergo testing for these allergen mixes and were meanwhile confirmed not to have asthma or other clinical allergies. Detailed demography of these asthmatics or non-asthmatic subjects showed that they came from different regions of China. Hence, our study population (serum samples) could be useful for evaluating the efficiency of Phadiatop test when regional specificity of allergen sensitization is taken into consideration. The diagnosis of asthma was based on the Global Initiative for Asthma (GINA) guidelines (12). In identifying the study population, we excluded sera from subjects with history of specific immunotherapy, immunodeficiency or parasitic infection. Finally, 382 serum samples from asthmatic patients (n=290, asthma group) or health checkup subjects (n=92, non-asthma group) were available for Phadiatop test.
Phadiatop tests
Eligible serum samples were retrieved from the biobank of AIR-SKLRD. These samples had been previously prepared by recovering the supernatant after a 10-minute centrifugation of 5 mL phlebotomized blood at 3,000 g, and placed in −80 °C refrigerators for long-term storage and future use.
All serum samples, with complete data of previous ImmunoCAP assays of house dusts mix (hx2), molds and yeasts mix (mx2), tree pollen mix (tx4) and weed pollen mix (wx5), were re-evaluated by Phadiatop test in the present study. The Phadiatop tests were performed using an ImmunoCAP 1000 system independently by a skillful investigator (N Wei) who was blinded of the previous detection results of the samples. Procedure instructions by the manufacturer were rigorously followed (13). The ImmunoCAP 1000 system automatically computed the total Phadiatop sIgE level (kUA/L) in each serum sample. Samples with a total Phadiatop sIgE level ≥0.35 kUA/L was reported qualitatively by the detection system as Phadiatop-positive, and those <0.35 kUA/L as Phadiatop-negative.
As an expedient to avoid confusion with Phadiatop findings, samples positive to any allergen mixes identified previously by ImmunoCAP assay were defined as allergen mix-positive; or hx2-, mx2-, tx4- and wx5-positive, if specified. The opposite was defined as allergen mix-negative; or hx2-, mx2-, tx4- and wx5-negative, if specified. Similarly, sIgE levels for hx2, mx2, tx4 or wx5 measured previously by ImmunoCAP assay was defined as allergen mix sIgE levels for the specified allergen mix. Allergen mix positivity to one of hx2, mx2, tx4 and wx5 alone was defined as mono-mix positivity, and to more than one of these allergen mixes, as poly-mix positivity.
Statistical analysis
Data were processed with a statistical software package SPSS 22.0 (IBM Corp, Armonk, NY, USA). Parametric quantitative data were presented as mean ± standard deviation (SD). Non-parametric quantitative data such as total Phadiatop sIgE levels were presented as median (25th and 75th percentiles). Categorical data were reported as percentage/proportion of positive results. Proportions were compared between groups with chi-square tests (χ2). F-tests were used to compare the variance of data among groups. Mann-Whitney U tests were used to compare non-parametric data. Correlation between non-parametric data was analyzed using Spearman’s tests, with correlation coefficients presented as “rs”. Diagnostic performance data of Phadiatop, including sensitivity, specificity, receiver operating characteristic (ROC) curve with area under the curve (AUC), positive or negative predictive values, accuracy, and positive or negative likelihood ratios, were calculated using the previous ImmunoCAP results as the reference standard. Cut-off values related to ROC were determined based on Youden index. P values <0.05 were considered statistically significant.
Results
Baseline and Phadiatop characteristics of the study samples
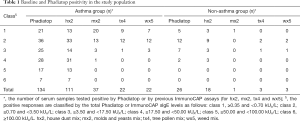
The study flow-chart is given in Figure 1. Prior to Phadiatop testing, 132 of serum samples from the asthma group (132/290, 45.5%) and 19 from the non-asthma group (19/92, 20.7%) were allergen mix-positive. In the whole study population or in either group, hx2-positivity accounted for majority of allergen mix-positive serum samples by overall prevalence or by higher-class (≥ class 4) responses (Table 1).
Full table
Among the 290 asthmatic patients, the overall rate of Phadiatop-positivity was 46.2% (n=134), comparable to the data by previous ImmunoCAP assays (45.5%). Although the donors of test sera came from different parts of China, there was no significant difference in sIgE positivity between Phadiatop and previous allergen mix tests for any individual regional source of serum samples (Figure 2A).
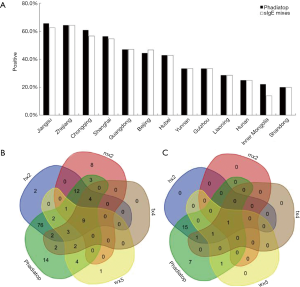
In the asthma group, there was a prominent overlap in allergen mix positivity. In these sera, Phadiatop tests demonstrated satisfactory sensitivity for tree pollen (tx4) (100%), house dusts (hx2) (98.2%) and weed pollen (wx5) (95.5%), but not for molds and yeasts (mx2) (78.4%). With regards to mono- vs. poly-mix positivity, Phadiatop identified 88.5% of 96 samples positive to one allergen mix alone, and 100% of all samples positive to two allergen mixes (n=18) and to three or four allergen mixes (n=17) (Figure 2B).
Among the non-asthma controls, the rate of Phadiatop positivity was 28.3%, which was slightly higher than 20.7% in the previous findings. All allergen mix-positive serum samples in this group were Phadiatop-positive (Figure 2C).
Total Phadiatop sIgE levels in relation to mono- and poly-mix positivity detected by previous ImmunoCAP assay
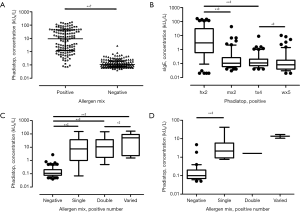
In the asthma group, 132 serum samples (45.5%) were allergen mix-positive; of them, 121 (91.7%) were Phadiatop-positive. Allergen mix-positive samples showed significantly higher mean level of total Phadiatop sIgE than allergen mix-negative ones [9.24 (1.12, 43.22) vs. 0.11 (0.08, 0.20) kUA/L, Mann-Whitney U =770.5, P<0.001] (Figure 3A). Moreover, samples positive to more allergen mixes were also associated with higher levels of total Phadiatop sIgE (Figure 3B). On the other hand, among the 134 Phadiatop-positive samples in the asthma group, the previous ImmunoCAP sIgE levels for hx2 [2.98 (0.60, 31.06) kUA/L] were significantly higher than those for mx2 [0.11 (0.06, 0.26) kUA/L], tx4 [0.11 (0.07, 0.21) kUA/L], and wx5 [0.08 (0.04, 0.17) kUA/L] (Kruskal-Wallis H =192.7, P<0.001) (Figure 3C). Similar findings were found for the non-asthma controls in that allergen mix-positive samples had higher total Phadiatop sIgE levels [2.68 (0.94, 10.57) kUA/L] than negative ones [0.10 (0.07, 0.20) kUA/L] (Mann-Whitney U =22.0, P<0.001), and that Phadiatop-positive samples had higher previous ImmunoCAP sIgE level for hx2 [0.85 (0.22, 3.72) kUA/L]. However, samples with mono-mix positivity and those with poly-mix positivity did not differ in total Phadiatop sIgE levels (P>0.05) (Figure 3D).
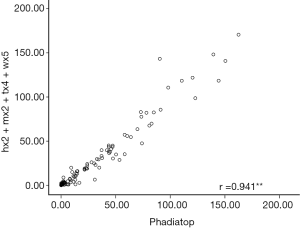
For all samples in the asthma group, a positive correlation was found between the total Phadiatop sIgE level and the sum of ImmunoCAP sIgE levels for the allergen mixes (hx2, mx2, tx4 and wx5 combined) (rs=0.941, P<0.001). In addition, the total Phadiatop sIgE level was also correlated with the ImmunoCAP sIgE levels for each allergen mix, particularly with hx2 sIgE level where the strongest correlation was found (rs=0.924, Figure 4) (all P<0.001).
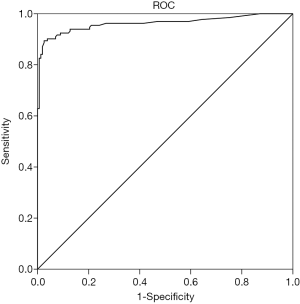
ROC curve analysis of Phadiatop testing
The diagnostic performance of Phadiatop testing was evaluated according to previous ImmunoCAP findings in the asthma group. This was based on the previous positivity or negativity for any allergen mix rather than for individual ones. The overall concordance rate was 91.7%; negative predictive value, 92.9%; and positive predictive value, 90.2%. ROC curve analysis of 132 allergen mix-positive and 158 negative serums indicated that, with 0.49 kUA/L and 0.35 kUA/L as the cut-off values, the Youden indices were 0.864 and 0.835, respectively. The Youden index was highest (0.869) using 0.53 kUA/L as the cut-off value, yielding a sensitivity of 89.4%, a specificity of 97.5%, with the AUC being 0.963 in identifying serums which were previously allergen mix-positive (Figure 5). If 0.53 kUA/L was used as the cut-off value, the positive and negative likelihood ratios were 35.76 and 0.03 respectively. In other words, of every 100 Phadiatop-negative serum samples, a maximum of 3 are likely to be false negative, whereas of every 100 Phadiatop-positive samples, at least 90 are likely to be positive, using the previous ImmunoCAP assay as gold standard.
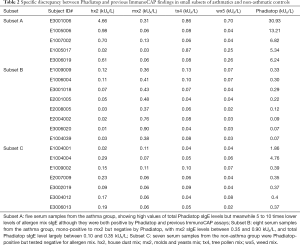
Specific discrepancy between Phadiatop and previous ImmunoCAP findings
Discrepancy between Phadiatop and previous ImmunoCAP findings was found among a small number of serum samples from the asthma or non-asthma group, based on the preset cut-off value (0.35 kUA/L). Five serum samples from the asthma group (5/290, 1.7%) showed considerably high total Phadiatop sIgE level in contrast to relatively low allergen mix sIgE levels (Table 2, Subset A). Although these samples were allergen mix-positive and meanwhile Phadiatop-positive, the total Phadiatop level in each sample was nearly 5 to 10 times greater than the sum or the highest value of all allergen-mix sIgE levels. Again, eight samples (8/290, 2.8%) from the asthma group were allergen mix-positive but tested negative on Phadiatop. Interestingly, these samples were mono-mix positive to mx2 alone and with a low allergen mix sIgE level between 0.35 and 0.90 kUA/L, and mostly with a low total Phadiatop sIgE levels between 0.10 and 0.35 kUA/L except for one sample with 0.09 kUA/L and two with 0.07 kUA/L (Table 2, Subset B). In the non-asthma group, seven samples were allergen mix-negative but positive by Phadiatop (Table 2, Subset C), whereas none of allergen mix-positive samples tested Phadiatop-negative.
Full table
Discussion
Evidence remains lacking with regard to the diagnostic efficiency of Phadiatop screening compared with “classic” ImmunoCAP assay for individual allergens or multi-allergen mixes among atopic patients (14), particularly in China. In this validation study using serum samples from a Chinese cohort coming from diverse parts of this country, satisfactory consistency was found in overall sIgE positivity between Phadiatop test and previous ImmunoCAP assay (46.2% vs. 45.5%) on allergen mixes among 290 asthmatic patients. Importantly, the consistency between the both detection was not interfered by places of residence where these serum contributors came from.
The accuracy of Phadiatop test in differentiating between allergic and non-allergic patients may be reliably up to 90% (15). In this study, based on the previous allergen mix-positivity as the gold standard, a single Phadiatop test identified inhalant allergen-sensitized subjects with 91.7% accuracy. Further, the accuracy of Phadiatop test was 100% for the subjects who had been sensitized to two or more allergen mixes, suggesting the usefulness of Phadiatop in screening subjects who are poly-sensitized to multiple allergens.
Usually, Phadiatop test is reported in a qualitative manner according to a cut-off value being 0.35 kUA/L as used in “classic” ImmunoCAP. Beyond the positivity or negativity of the Phadiatop testing, it remains rarely studied whether the total Phadiatop sIgE levels suggest any clinical relevance. Interestingly, in serum samples from these Chinese patients with asthma, we demonstrated the total Phadiatop sIgE level was significantly correlated with the sum of ImmunoCAP sIgE levels for the allergen mixes, or ImmunoCAP sIgE levels of each allergen mix, in particularly, of hx2 (rs=0.924). These findings suggest that higher levels of total Phadiatop sIgE may be associated with greater likelihood of sensitization to common aeroallergens. While the cut-off value of 0.35 kUA/L is recommended by the ImmmoCAP manufacturer and has been widely adopted in clinical laboratories, a number of studies have justified the need to use other cut-off values (0.12 to 0.43 kUA/L) for screening tests according to different target allergens and specific populations (16-18). By ROC analysis, we demonstrated that using a cut-off value of 0.53 kUA/L, Phadiatop identified atopy in serum samples (any allergen-mix positivity) from asthmatics with a sensitivity of 89.4% and a specificity of 97.5%, respectively, with a greatest AUC of 0.963.
Previous studies revealed favorable performance with Phadiatop test in screening sensitization to most of inhalant allergens while using a tiny volume of serum (40 µL) in a single detection (8,19). In the present study, Phadiatop testing showed high sensitivity for tx4 (100%), hx2 (98.2%), and wx5 (95.5%), and also well identified weak-positive serum samples with low ImmunoCAP sIgE levels (0.35–0.70 kUA/L, corresponding to class 1 sIgE response) for these three allergen mixes. In contrast, the sensitivity by Phadiatop test was unsatisfactory (78.4%) for mx2-positive serum samples. Yet, all the Phadiatop-missed cases (mx2-positive but Phadiatop-negative samples, n=8) were from the asthma group, mono-positive to mx2 and with very low mx2 sIgE levels (>0.35 and ≤0.90 kUA/L, corresponding to sIgE response of class 1 or slightly higher). For these missed cases, the total Phadiatop sIgE levels largely ranged between 0.10 and 0.35 kUA/L. It should be noteworthy that these were based on the Phadiatop cut-off value of 0.35 kUA/L. The sensitivity of Phadiatop test for mx2 in this study, however, would be 91.8% when judging by a total Phadiatop sIgE level of 0.10 kUA/L. Given that nearly 25% of Chinese territory is located in subtropical regions where the natural environments and climates favor growth of molds, we propose the need for subsequent targeted test using ImmunoCAP assay for mold and yeast among Phadiatop-negative subjects whose total Phadiatop sIgE levels were between 0.10 and 0.35 kUA/L, particularly for asthma patients in this country.
An efficient diagnostic strategy could substantially contribute to identification of subjects with atopy, hence better prevention and management of allergic disorders with less medical cost. This can be of paramount relevance, particularly in a developing country with limited medical budgets. ImmunoCAP techniques as the gold standard for in vitro allergen sIgE tests have been well established and available worldwide. However, not paralleled with the increasing prevalence of allergies and asthma in China (20), little attention has been drawn to reflect on the cost-effectiveness of conventional strategy via sequential testing of allergen mixes or individual allergens (7,19,21). We conducted this study expecting to inspire future perspective on this topic. Interestingly, in our study, there were five samples from the asthma group showing high Phadiatop sIgE levels but low allergen mixes sIgE levels, and seven samples from the non-asthma group testing positive by Phadiatop but negative by ImmunoCAP for all the studied allergen mixes. We speculated that Phadiatop positivity in these samples may suggest sensitization to other more aeroallergens beyond the four allergen mixes in this study (hx2, mx2, tx4 and wx5). This may suggest a potential advantage of using a single Phadiatop test as first-line test over “classic” ImmunoCAP assays for allergen mix combinations in asthma subjects with suspected atopy. Determination on ImmunoCAP test panel of allergen mixes highly relies on physician expertise, patient history, socioeconomical status and local epidemiology. Due to variations in these aspects, the size of an ImmunoCAP test panel may be sometimes too small or large (including too few or many allergens or allergen mixes for screening), which potentially results in missed diagnoses or excessive medical costs regardless of “classic” ImmunoCAP assays being the gold standard of in-vitro allergen sIgE detection.
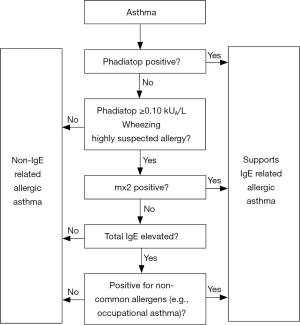
While GINA guidelines emphasize on the importance of allergen tests in asthma, no recommendations are specifically given on the strategy of allergen testing. The guidelines state that skin test is as efficient as in-vitro sIgE test but the latter may be preserved for uncooperative patients, or those at risk of anaphylaxis. Although widely accessible, skin tests necessitate the use of non-standardized, crude allergen extracts which may occasionally contain other sensitizing components that confound the outcome interpretation. The reliability of the skin test also critically depends on skillful manipulation by allergists. An even more important limitation for skin tests is the extremely rigorous and complicated, if not laggard, procedure for registration and approval of crude extract products used for skin test in China. As a result, currently there have been only two products (Dermatophagoides pteronyssinus and Dermatophagoides farina) officially approved by the China Food and Drug Administration for used in skin tests. Considerably, these technical and regulatory factors do not favor the use of skin tests for diagnosing atopy in the Chinese population, although skin tests are much cheaper. Based on our findings in this study, we preliminarily proposed an algorithm for screening sensitization to inhalant allergens among Chinese asthmatics, using Phadiatop as first-line step of testing (Figure 6). Briefly, asthma patients screened positive on Phadiatop could be sequentially tested for dust mites and pollen. Those who are highly suspected to have atopy but with negative Phadiatop results could undergo further tests for molds and yeast. We speculated that such an algorithm may have implications in terms of medical costs for China and other countries with limited medical budgets. As a clinical practice in China, at least five allergen mixes (hx2, mx2, tx4, wx5 and ex1) need to be tested as a first-line testing when asthma is suspected to be of an allergic nature. Each test for allergen mix test costs ~4.3 USD, adding up to a total expense of 21.5 USD per patient, in a drastic contrast to only 4.3 USD by using a single Phadiatop test. For China with a large population (1,390 million as of 2017) and a roughly 5% prevalence of asthma, this could translate into a reduction of medical cost by approximately 120 million USD using Phadiatop as first-line screening test of aeroallergens. Regardless of the supplementary allergen tests which may follow a positive Phadiatop screening, the medical costs would nevertheless be much lower. In this regard, Phadiatop test could be useful for its cost-effectiveness in allergen tests.
There are several limitations in this study. Firstly, from a pragmatic point of view, we focused on four common inhalant allergen mixes (hx2, mx2, tx4, wx5) reported in Chinese populations, without inclusion of more other allergen mixes (e.g., ex1, gx6). Whereas our validation study is clinically relevant, we acknowledge that the profiles of common allergens causing sensitization could be different elsewhere, rendering further studies needed. Secondly, we focused on the use of Phadiatop among patients with asthma but not others like eczema or allergic dermatitis, because asthma is frequently caused by sensitization to inhalant allergens while eczema or allergic dermatitis may sometimes arise from non-sIgE response or food allergens. Therefore, this study would not be able to shed light on the value of Phadiatop in a general population. Moreover, as we have stated that Phadiatop test is not designed for screening of chemicals, drugs or food allergens, the benefit of Phadiatop should not be extrapolated beyond the spectrum of inhalant allergens. Future studies are inspired to address these questions.
Conclusions
In summary, Phadiatop test showed satisfactory diagnostic efficacy for detecting sensitization to four common inhalant allergen mixes in this Chinese cohort of asthma patients using ImmunoCAP as the gold standard. Using a new cut-off value may optimize the efficiency of Phadiatop in identifying serum samples positive to any of these allergen mixes. Discrepancy between Phadiatop and ImmunoCAP for mx2 detection suggested the need for subsequent testing of molds when total Phadiatop sIgE levels were between 0.10 and 0.35 kUA/L. In light of the currently rigorous administration on crude extracts for skin tests in China, using Phadiatop as a first-line test for subjects with suspected atopy can be cost-effective. Our findings may add to strategy about allergy diagnosis in China and worldwide.
Acknowledgements
Funding: This study was supported by National Natural Science Foundation of China (NSFC 81572063; NSFC 81601394); Guangdong Science and Technology Foundation (2014A020212352); the Guangzhou Education Bureau (1201630044; 1201630393); and State Key Laboratory of Respiratory Disease Foundation (SKLRD20160P017; 2014SRLRD-008).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of First Affiliated Hospital of Guangzhou Medical University (approval number: GYFYY-2016-73). The use of human serum samples was in accordance with the legislation in China and the wishes of donors, their legal guardians or next of kin, where applicable, who had offered written informed consent to using the serum samples for future unspecified research purposes.
References
- Blackley CH. Experimental Researches on the Causes and Nature of Catarrhus Aestivus. London: Baillière, 1873.
- Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 1971;8:871-4. [Crossref] [PubMed]
- Sastre J. Molecular diagnosis in allergy. Clin Exp Allergy 2010;40:1442-60. [Crossref] [PubMed]
- Wood RA, Segall N, Ahlstedt S, et al. Accuracy of IgE antibody laboratory results. Ann Allergy Asthma Immunol 2007;99:34-41. [Crossref] [PubMed]
- Wang J, Godbold JH, Sampson HA. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol 2008;121:1219-24. [Crossref] [PubMed]
- Leimgruber A, Mosimann B, Claeys M. Clinical evaluation of a new in-vitro assay for specific IgE, the immuno CAP system. Clin Exp Allergy 1991;21:127-31. [Crossref] [PubMed]
- Tong S, Berry HL, Ebi K, et al. Climate change, food, water and population health in China. Bull World Health Organ 2016;94:759-65. [Crossref] [PubMed]
- Williams PB, Siegel C, Portnoy J. Efficacy of a single diagnostic test for sensitization to common inhalant allergens. Ann Allergy Asthma Immunol 2001;86:196-202. [Crossref] [PubMed]
- Luo W, Huang H, Zheng P, et al. Major grass pollen allergens and components detected in a southern Chinese cohort of patients with allergic rhinitis and/or asthma. Mol Immunol 2016;78:105-12. [Crossref] [PubMed]
- Sun B, Zheng P, Wei N, et al. Co-sensitization to silkworm moth (Bombyx mori) and 9 inhalant allergens among allergic patients in Guangzhou, southern China. PLoS One 2014;9:e94776. [Crossref] [PubMed]
- Zeng G, Luo W, Zheng P, et al. Component-resolved diagnostic study of Dermatophagoides pteronyssinus major allergen molecules in a southern Chinese cohort. J Investig Allergol Clin Immunol 2015;25:343-51. [PubMed]
- Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008;31:143-78. [Crossref] [PubMed]
- Thermo Scientific. Available online: http://www.phadia.com/
- de Waard AH, Peters EM. Phadiatop testing in assessing predisposition to respiratory tract symptoms of allergic origin in athletes. S Afr Med J 2012;102:309-11. [Crossref] [PubMed]
- Twaroch TE, Curin M, Valenta R. Mold allergens in respiratory allergy: from structure to therapy. Allergy Asthma Immunol Res 2015;7:205-20. [Crossref] [PubMed]
- Ebo DG, Venemalm L, Bridts CH, et al. Immunoglobulin E antibodies to rocuronium: a new diagnostic tool. Anesthesiology 2007;107:253-9. [Crossref] [PubMed]
- Holt PG, Rowe J, Kusel M, et al. Toward improved prediction of risk for atopy and asthma among preschoolers: a prospective cohort study. J Allergy Clin Immunol 2010;125:653-9. [Crossref] [PubMed]
- Linden CC, Misiak RT, Wegienka G, et al. Analysis of allergen specific IgE cut points to cat and dog in the Childhood Allergy Study. Ann Allergy Asthma Immunol 2011;106:153-158.e2. [Crossref] [PubMed]
- Garcia-Marcos L, Sanchez-Solis M, Martinez-Torres AE. Phadiatop compared to skin-prick test as a tool for diagnosing atopy in epidemiological studies in school children. Pediatr Allergy Immunol 2007;18:240-4. [Crossref] [PubMed]
- Denholm R, Crellin E, Arvind A. Asthma and lung cancer, after accounting for co-occurring respiratory diseases and allergicconditions: a systematic review protocol. BMJ Open 2017;7:e013637. [Crossref] [PubMed]
- Hwang H, Kwon J, Kim J Y, et al. The RIDA allergy screen versus the Phadiatop test in 430 consecutive patient specimens. Lab Med 2016;47:20-9. [Crossref] [PubMed]


