Rosiglitazone inhibits PM2.5-induced cytotoxicity in human lung epithelial A549 cells
Introduction
Air pollution is nowadays regarded as a major threat to public health worldwide. Particular matter (PM) is a significant class of air pollutants, consisting of PM10, PM2.5 and ultrafine PM based on the sizes and aerodynamic properties. PM2.5 refers to the particles and droplets with aerodynamic diameter ≤2.5 µi (1). Increasing evidences have shown that long-term exposure to PM2.5 is epidemiologically associated with respiratory diseases (2,3). Respiratory system is the direct organ contact with ambient PM2.5, and thus airway cells suffer more damage than other cells. As reported, PM2.5 exposure causes cell membrane lysis and mitochondrial ultrastructural disruptions in lung alveolar epithelial cells (A549) (4). In addition, PM2.5 induces DNA damage and inflammatory response, and meanwhile increases the generation of reactive oxygen species (ROS) in A549 cells (5-11), which are the major mechanisms for PM2.5-induced cell injury (12).
Peroxisome proliferator-activated receptor gamma (PPARγ) is a ligand-dependent transcription factor, and a member of nuclear receptor of peroxisome proliferator-activated receptors (PPARs) family. PPARs have multiple regulatory roles in inflammation, redox balance, trophic factor biosynthesis, insulin sensitivity and metabolism of lipid and glucose. Rosiglitazone, one of the agonists of PPARγ, is a thiazolidinedione drug used in the treatment of type 2 diabetes mellitus (13). In recent years, the resistance function of rosiglitazone in cell apoptosis, inflammation response and oxidative stress have been verified (14-17). However, whether rosiglitazone is able to prevent cell injury and death in lung epithelial cells resulted from PM2.5 still remains unclear.
In the present work, we firstly found decreasing of PPARγ in PM2.5-treated A549 cells. Then using a PPARγ agonist, rosiglitazone, we confirmed that PPARγ activation suppressed cell apoptosis and ROS production in A549 cells exposed to PM2.5. Our results further demonstrated that rosiglitazone inhibited ERK1/2 and STAT3 activated by PM2.5 exposure. Our data collectively suggested that rosiglitazone can protect human lung epithelial cells from cell toxicity of PM2.5.
Methods
Preparation of PM2.5
Fifty mg PM2.5 (National Institute of Standards and Technology Boulder Laboratories, Boulder, CO, USA) was dissolved in 1 mL dimethyl sulfoxide (DMSO) facilitated with 20 min ultrasonic oscillation. The mixture was centrifuged at 17,000 g and stored at 4 °C for 10 min.
Cell culture and treatment
Human lung alveolar epithelial A549 cell was a gift from Cell Bank, Chinese Academy of Sciences. Cells were routinely maintained in DMEM supplemented with 10% fetal bovine serum (FBS, Biological Industries, Israel) at a 5% CO2 atmosphere at 37 °C. A549 cells were seeded and treated with prepared PM2.5 at the final concentration of 25 or 50 µg/mL for 48 h. Rosiglitazone (Selleck) was incubated at a final concentration of 20 µM with A549 cells for 48 h.
Measurement of cellular apoptosis
Cellular apoptosis was accessed with flow cytometry according to the manufacturer’s protocols of FITC Annexin V Apoptosis Detection Kit I (BD, Pharmingen, USA). Simply, A549 cells in 1X Binding Buffer were incubated in dark with FITC Annexin V and PI for 15 min at room temperature. Then the cells were analyzed by flow cytometry immediately.
Western blot
After treatment with PM2.5 or Rosiglitazone, A549 cells were harvested and lysed on ice in cell lysis buffer (KeyGen, China) supplied with 1 mM PMSF (KeyGen). The concentration of protein was quantified by BCA Protein Assay Kits (Takara, Japan). Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, USA) by electricity. After incubation with 5% BSA (KeyGen), membranes were then incubated with primary antibodies at 4 °C overnight: Bax (Abclonal, USA), Bcl2 (Abclonal), PPARγ (Abclonal), P-ERK1/2 (Abclonal), ERK1/2 (Abclonal), P-STAT3 (Bioworld, USA), STAT3 (Bioworld), GAPDH (Abclonal). After that, the membranes were further incubated with appropriate secondary antibodies for 2 h. The protein signals were visualized and recorded using the ECL plus western blotting detection reagents (Millipore) and the ChemiDoc XRS Plus luminescent image analyzer (Tanon).
Measurement of intracellular oxidative stress
After treated with PM2.5 or rosiglitazone, the cells were harvested and resuspended in pre-warmed media with 10 µM DCFH-DA (Beyotime, China) and incubated for 30 min. After washing with PBS, the DCFH-DA fluorescence was measured using flow cytometric analysis (Beckman) by filtering 488 nm. And the mean fluorescence intensity was quantitated.
Statistical analysis
Results were all expressed as mean ± SEM. Unpaired two-tailed Student’s t-test was applied for comparisons between two groups. One-way ANOVA followed by Bonferroni’s post hoc test was conducted when comparing among multiple groups. All analyses were carried out with GraphPad Prism 6.0. P value less than 0.05 were considered statistically significant.
Results
PPARγ is decreased in PM2.5-treated A549 cells
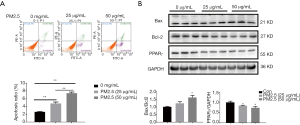
To mimic lung epithelial cell injury due to PM2.5 exposure, A549 cells were treated with 25 and 50 µg/mL PM2.5. Results demonstrated that 48 h of PM2.5 treatment led to potent cell apoptosis in A549 cells, as determined by flow cytometry analysis (Figure 1A) and protein level for Bax and Bcl2 (Figure 1B). Furthermore, western blot also showed that PPARγ was significantly downregulated in PM2.5-stimulated A549 cells (Figure 1B). In addition, a dose-dependent increase of cell apoptosis and decrease of PPARγ expression were also observed (Figure 1). Thus, reduction of PPARγ might be responsible for lung alveolar epithelial damage induced by PM2.5 exposure.
Rosiglitazone attenuates PM2.5-induced apoptosis in A549 cells
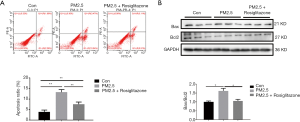
In order to further examine the functional effect of PPARγ on lung epithelial cells during PM2.5 exposure, rosiglitazone was used as the PPARγ agonist. Flow cytometry suggested that rosiglitazone reduced PM2.5-mediated apoptosis in A549 cells (Figure 2A). Meanwhile, rosiglitazone reduced the expression of Bax/Bcl2 ratio as determined by western blot (Figure 2B). These results suggested the protective effects of rosiglitazone against apoptosis in human lung epithelial cells caused by PM2.5 treatment.
Rosiglitazone attenuates PM2.5-induced ROS production in A549 cells
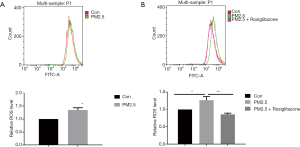
Oxidative stress has been reported as a crucial pattern of cell toxicity induced by PM2.5. DCFH-DA was used to quantify the intracellular level of ROS. As shown in flow cytometry results, PM2.5 exposure elevated ROS production in cells (Figure 3A), and rosiglitazone significantly reduced ROS production in cells with PM2.5 exposure (Figure 3B). Thus, these data suggested that rosiglitazone protects against PM2.5-caused cell toxicity in human lung epithelial cells through reducing ROS generation.
Rosiglitazone inhibits PM2.5-activated ERK1/2 and STAT3 signaling in A549 cells
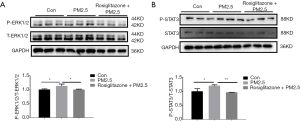
Given that ERK1/2 and STAT3 signaling pathways play critical roles in regulating cell apoptosis and oxidative stress (18-21), we next investigated the effect of rosiglitazone and PM2.5 on activation of ERK1/2 and STAT3. Our results showed that PM2.5 exposure increased the protein level of phosphorylated ERK1/2 and STAT3, whereas rosiglitazone suppressed the upregulated phosphorylated ERK1/2 and STAT3 in PM2.5-treated cells (Figure 4A,B). These data revealed that ERK1/2 and STAT3 inactivation might be responsible for the protective action of rosiglitazone in A549 cells during PM2.5 exposure.
Discussion
Urbanization and associated air pollution, in particular, PM2.5 increases the morbidity of various respiratory diseases (22). It is well-known that PM exposure played detrimental roles mostly in epithelial cells (4,22). However, the underlying molecular mechanism is largely unknown and the effective therapeutic methods are still lack. In present work, we verified that PM2.5 mediated ROS generation and cell apoptosis in A549 cells. Importantly, our results demonstrated for the first time that PPARγ agonist rosiglitazone could protect against PM2.5-mediated A549 cell apoptosis and oxidative stress accumulation through inhibiting ERK1/2 and STAT2 signaling pathways.
Previous studies have linked several genes to airway epithelial cell damage induced by PM2.5, which exerting their roles through regulating cell death and inflammatory response (23-27). It is well-accepted that PPARγ is widely expressed in almost all kinds of human tissues and plays central roles in early stage lung development (28-30). PPARγ agonists prevent newborn mouse from hypoxia-induced lung injury (31). Particularly, PPARγ has been shown decreased in hepatic stellate cells exposed to PM2.5 during liver fibrosis (32). However, the expression changes and roles of PPARγ in respiratory systems exposed to PM2.5 are still unknown. This is the first study that reveals the downregulation and the protective roles of PPARγ in A549 cells treated with PM2.5. Given that PPARγ deficiency is widely associated with pulmonary disorders including, asthma, cystic fibrosis lung cancer (29). Our results indicated that PM2.5 exposure might lead to these pulmonary diseases through reducing the PPARγ expression. Our results also suggested that PPARγ activation is an alternative therapeutic strategy for treatment of lung injury caused by PM2.5.
ROS has diverse effects on cellular activities depending on its amount. In physiological conditions, the lung epithelial cells generate low levels of ROS and physiological changes of ROS levels are essential for cell survival. PPARγ agonist GW1929 elevated ROS levels and activated cell-cycle progression in primary cultured lung alveolar epithelial cells (33). However, in pathological conditions, prolonged oxidative stress leads to cell apoptosis and death. Herein, we found that PPARγ agonist rosiglitazone reduced ROS levels and inhibited the lung epithelial cell apoptosis during PM2.5 exposure. Thus, PPARγ might have different roles in ROS production due to the physiological and pathological conditions.
Numerous studies have suggested a central role of ERK1/2 and STAT3 signaling pathways in regulating oxidative stress and related cell apoptosis (18-21). As expected, phosphorylation of ERK1/2 and STAT3 were increased after PM2.5 exposure. Importantly, rosiglitazone stimulation repressed the activation of ERK1/2 and STAT3 pathways. Although further studies are required to clarify the detailed molecular mechanisms, results of present work indicated that rosiglitazone could protect against PM2.5-mediated cell damage via inhibiting ERK1/2 and STAT3 pathways.
Conclusions
In summary, our work demonstrated that PPARγ was downregulated in A549 cells exposed to PM2.5. Application of rosiglitazone, a PPARγ agonist, could relieve the cytotoxic effect of PM2.5 on human lung epithelial A549 cells by inhibiting ERK1/2 and STAT3 activation. Our results suggested rosiglitazone as a potential therapeutic agent of PM2.5-induced lung diseases.
Acknowledgements
Funding: This study was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS, 2016-12M-1-006).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ma QY, Huang DY, Zhang HJ, et al. Exposure to particulate matter 2.5 (PM2.5) induced macrophage-dependent inflammation, characterized by increased Th1/Th17 cytokine secretion and cytotoxicity. Int Immunopharmacol 2017;50:139-45. [Crossref] [PubMed]
- Liu F, Huang Y, Zhang F, et al. Macrophages treated with particulate matter PM2.5 induce selective neurotoxicity through glutaminase-mediated glutamate generation. J Neurochem 2015;134:315-26. [Crossref] [PubMed]
- Yang D, Ma M, Zhou W, et al. Inhibition of miR-32 activity promoted EMT induced by PM2.5 exposure through the modulation of the Smad1-mediated signaling pathways in lung cancer cells. Chemosphere 2017;184:289-98. [Crossref] [PubMed]
- Gualtieri M, Mantecca P, Corvaja V, et al. Winter fine particulate matter from Milan induces morphological and functional alterations in human pulmonary epithelial cells (A549). Toxicol Lett 2009;188:52-62. [Crossref] [PubMed]
- Wang W, Jariyasopit N, Schrlau J, et al. Concentration and photochemistry of PAHs, NPAHs, and OPAHs and toxicity of PM2.5 during the Beijing Olympic Games. Environ Sci Technol 2011;45:6887-95. [Crossref] [PubMed]
- Cavanagh JA, Trought K, Brown L, et al. Exploratory investigation of the chemical characteristics and relative toxicity of ambient air particulates from two New Zealand cities. Sci Total Environ 2009;407:5007-18. [Crossref] [PubMed]
- Bourgeois B, Owens JW. The influence of Hurricanes Katrina and Rita on the inflammatory cytokine response and protein expression in A549 cells exposed to PM2.5 collected in the Baton Rouge-Port Allen industrial corridor of Southeastern Louisiana in 2005. Toxicol Mech Methods 2014;24:220-42. [Crossref] [PubMed]
- Lin ZQ, Xi ZG, Yang DF, et al. Oxidative damage to lung tissue and peripheral blood in endotracheal PM2.5-treated rats. Biomed Environ Sci 2009;22:223-8. [Crossref] [PubMed]
- Corsini E, Budello S, Marabini L, et al. Comparison of wood smoke PM2.5 obtained from the combustion of FIR and beech pellets on inflammation and DNA damage in A549 and THP-1 human cell lines. Arch Toxicol 2013;87:2187-99. [Crossref] [PubMed]
- Yan Z, Wang J, Li J, et al. Oxidative stress and endocytosis are involved in upregulation of interleukin-8 expression in airway cells exposed to PM2.5. Environ Toxicol 2016;31:1869-78. [Crossref] [PubMed]
- He M, Ichinose T, Yoshida S, et al. PM2.5-induced lung inflammation in mice: Differences of inflammatory response in macrophages and type II alveolar cells. J Appl Toxicol 2017;37:1203-18. [Crossref] [PubMed]
- Deng X, Zhang F, Rui W, et al. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol In Vitro 2013;27:1762-70. [Crossref] [PubMed]
- Sagir D, Eren B, Yilmaz BD, et al. Effects of prenatal PPAR-gamma agonist rosiglitazone exposure on rat hippocampus development in a time-dependent manner: A stereological and histopathological study. Hum Exp Toxicol 2017. [Epub ahead of print]. [PubMed]
- Lee JE, Park JH, Jang SJ, et al. Rosiglitazone inhibits chlorpyrifos-induced apoptosis via modulation of the oxidative stress and inflammatory response in SH-SY5Y cells. Toxicol Appl Pharmacol 2014;278:159-71. [Crossref] [PubMed]
- Zhao J, Liu J, Pang X, et al. Rosiglitazone attenuates angiotensin II-induced C-reactive protein expression in hepatocytes via inhibiting AT1/ROS/MAPK signal pathway. Int Immunopharmacol 2016;31:178-85. [Crossref] [PubMed]
- An Z, Yu JR, Park WY. Rosiglitazone enhances radiosensitivity by inhibiting repair of DNA damage in cervical cancer cells. Radiat Environ Biophys 2017;56:89-98. [Crossref] [PubMed]
- Gong Y, Yin JY, Tong BD, et al. Low density lipoprotein - rosiglitazone - chitosan-calcium alginate/nanoparticles inhibition of human tenon's fibroblasts activation and proliferation. Oncotarget 2017;8:105126-36. [Crossref] [PubMed]
- Tian Y, Xiao Y, Wang B, et al. Vitamin E and Lycopene Reduce Coal Burning Fluorosis-induced Spermatogenic Cell Apoptosis via Oxidative Stress-mediated JNK and ERK Signaling Pathways. Biosci Rep 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Zhou XC, Dong SH, Liu ZS, et al. Regulation of gammaherpesvirus lytic replication by endoplasmic reticulum stress-induced transcription factors ATF4 and CHOP. J Biol Chem 2018;293:2801-14. [Crossref] [PubMed]
- Rai A, Kumar U, Raj V, et al. Novel 1,4-benzothazines obliterate COX-2 mediated JAK-2/STAT-3 signals with potential regulation of oxidative and metabolic stress during colorectal cancer. Pharmacol Res 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Rajamanickam V, Zhu H, Feng C, et al. Novel allylated monocarbonyl analogs of curcumin induce mitotic arrest and apoptosis by reactive oxygen species-mediated endoplasmic reticulum stress and inhibition of STAT3. Oncotarget 2017;8:101112-29. [Crossref] [PubMed]
- Yang B, Chen D, Zhao H, et al. The effects for PM2.5 exposure on non-small-cell lung cancer induced motility and proliferation. Springerplus 2016;5:2059. [Crossref] [PubMed]
- Zhou W, Yuan X, Zhang L, et al. Overexpression of HO-1 assisted PM2.5-induced apoptosis failure and autophagy-related cell necrosis. Ecotoxicol Environ Saf 2017;145:605-14. [Crossref] [PubMed]
- Nam HY, Choi BH, Lee JY, et al. The role of nitric oxide in the particulate matter (PM2.5)-induced NFkappaB activation in lung epithelial cells. Toxicol Lett 2004;148:95-102. [Crossref] [PubMed]
- Wang H, Guo Y, Liu L, et al. DDAH1 plays dual roles in PM2.5 induced cell death in A549 cells. Biochim Biophys Acta 2016;1860:2793-801. [Crossref] [PubMed]
- Song L, Li D, Gu Y, et al. Let-7a modulates particulate matter (≤ 2.5 µm)-induced oxidative stress and injury in human airway epithelial cells by targeting arginase 2. J Appl Toxicol 2016;36:1302-10. [Crossref] [PubMed]
- Huang Q, Chi Y, Deng J, et al. Fine particulate matter 2.5 exerted its toxicological effect by regulating a new layer, long non-coding RNA. Sci Rep 2017;7:9392. [Crossref] [PubMed]
- Rubenstrunk A, Hanf R, Hum DW, et al. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta 2007;1771:1065-81. [Crossref] [PubMed]
- Heikkinen S, Auwerx J, Argmann CA. PPARgamma in human and mouse physiology. Biochim Biophys Acta 2007;1771:999-1013. [Crossref] [PubMed]
- Wang Y, Santos J, Sakurai R, et al. Peroxisome proliferator-activated receptor gamma agonists enhance lung maturation in a neonatal rat model. Pediatr Res 2009;65:150-5. [Crossref] [PubMed]
- Takeda K, Okamoto M, de Langhe S, et al. Peroxisome proliferator-activated receptor-g agonist treatment increases septation and angiogenesis and decreases airway hyperresponsiveness in a model of experimental neonatal chronic lung disease. Anat Rec (Hoboken) 2009;292:1045-61. [Crossref] [PubMed]
- Zheng Z, Zhang X, Wang J, et al. Exposure to fine airborne particulate matters induces hepatic fibrosis in murine models. J Hepatol 2015;63:1397-404. [Crossref] [PubMed]
- Tickner J, Fan LM, Du J, et al. Nox2-derived ROS in PPARgamma signaling and cell-cycle progression of lung alveolar epithelial cells. Free Radic Biol Med 2011;51:763-72. [Crossref] [PubMed]


