A technique of hand digit reconstruction using scarred and deformed donor segments
Introduction
Transposition of fingers, finger stumps and metacarpal bones of the injured hand has a long history, however there are several factors that preclude its broader use in modern reconstructive surgery (1-3). A significant limitation is high likelihood of ischemic complications in cases when scarred and deformed donor segments are used. Vascular disorders lead to partial or complete necrosis of skin flaps and transferred segments in 11% of cases, even when the tissue is almost or completely intact (4-6). Transfer of scarred donor segments is often ruled out because the existing prevention methods for these complications are inefficient. Therefore, such operations are carried out only for certain hand defects with intact digital arteries, satisfactory condition of soft tissues and intact bony frame in the donor segment (2,7). The preferred solution among surgeons is often to transfer the mutilated finger or its stump—which is a “less complicated procedure” usually involving the II finger or the finger adjacent to the reconstructed one—in order to mitigate vascular disorder presentation in the transformed segment by minimizing the trauma of neurovascular bundles and transferred skin flaps. Unfortunately, it is impossible to transfer either short stumps of fingers and metacarpal bones that are remote from the receiving area, or deformed fingers in cases of major vessel damage and severely impaired tissue blood flow (3,8). These factors restrict the use of transposition, which could otherwise present new opportunities for improving outcomes of digit reconstruction. This outlines the need for the development and physiological validation of new approaches to improving macro- and microcirculation in scarred donor segments and preventing ischemic complications in reconstructed digits. The aim of this study was to investigate the opportunities for expanding the set of indications for hand digit reconstruction using scarred and deformed donor segments.
Methods
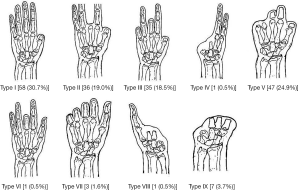
The study was approved by the local ethics committee (NNIITO-2012/12/28) and all participants gave informed consent. We analysed treatment outcomes of 184 patients [161 men (87.5%) and 23 women (12.5%)] who, in total, underwent primary and secondary reconstruction of 203 thumbs and fingers in 189 hands by transferring various segments of the injured hand using traditional and non-traditional techniques in 85 and 118 reconstructive procedures respectively. The most common type of injury was mechanical [n=101 (54.9%)], followed by frostbites [n=30 (16.3%)], damage after burns [n=27 (14.7%)], gunshot wounds [n=23 (12.5%)], and complex trauma [n=3 (1.6%)]. Right hand was damaged in 80 patients (43.5%), left hand—68 patients (37.0%); 36 patients had digit and hand stumps on both sides (19.6%). The types of injury were as follows: 100—home injuries (54.3%); 79—occupational injuries (42.9%); and 5—military injuries (2.7%). Figure 1 depicts the 9 types of hand defects observed and Table 1 shows the breakdown according to transferred segments in the study cohort. Amputation levels of reconstructed digits are presented in Table 2.

Full table
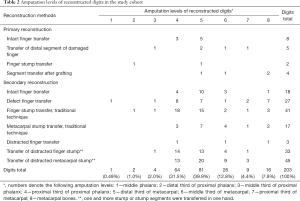
Full table
To reconstruct thumbs, the segments of the first radius were only transferred along the axis into a soft cage that had been formed with a skin flap. The donor material exhibited marked anatomical, morphological, and functional tissue polymorphism. Most donor segments (defect fingers, finger and metacarpal stumps) had cicatricially changed tissue with various degrees of scarring [n=166 (90.2%)]. This factor caused or significantly aggravated segment deformation, disruptions of function and blood flow, which dramatically increased the likelihood of ischemic complications. To overcome this limitation, we developed a technique involving seven main stages (Table 3).

Full table
The rationale to conduct research in this area is based on fundamental studies of the efficacy of ischemic preconditioning of organs (9) in cases of vascular pathologies, a technique that can also be applied for prevention and treatment of ischemic tissue disorders in surgery. To investigate the mechanisms of compensatory vascular response formation and opportunities for applying this approach in transferring of pathologically changed hand segments, we employed clinical, radiological, morphological, biophysical (IR imaging, laser Doppler flowmetry, rheovasography) methods.
We studied the effects of ischemic load of varying duration and frequency on the condition of micro- and macrocirculation in donor fingers. After signing an informed consent form, all patients exposed themselves to periodic local ischemia by applying an arteriovenous tourniquet on the II finger of the left hand four times a day using tight distal-proximal exsanguination type bandaging and leaving the distal phalanx free. The exposures ranged from 10 to 30 minutes (10). We used Thermo Tracer TH-9100 matrix thermal imager (NEC, Japan) to register the temperature dynamics on the dorsal surfaces of both hands in 54 experiments in 6 male volunteers (mean age 29 years) before, during and after the application of tourniquet.
The IR data was analyzed using the GTS 5.1.1.011 IR image processing software. IR maps processing included distortion compensation and sequence segmentation of thermal images (11). To measure the blood flow parameters in the finger under ischemic training and of the scarred donor segments, we used RGPA-6/12 “Rhean-Poly” 6-channel rheograph-polyanalyzer (Medicom MTD, Russia). To determine dermal microcirculation indices, we used LAKK-M laser Doppler flowmeter (Lazma, Russia). Wavelet transformation was used to analyze the amplitudes of oscillations in the ranges corresponding to active and passive factors of microvascular blood flow regulation (12). Cluster analysis and analysis of variance, correlation and regression were run in STATISTICA-10 software.
Results
The key study endpoint in the early postoperative period was segment acceptability. All segments, including the most severely damaged, were accepted after primary reconstruction. Complications after secondary reconstruction included necrosis of the whole transferred finger stump (n=1), partial necrosis of the distal phalanx of transferred mutilated finger (n=1), partial necrosis of soft tissues on the dorsal finger surface (n=1) and of the finger stump (n=1). Ischemic complications were observed in all cases of transfer of scarred and deformed segments, mostly—venous drainage disorders. In cases of transfer of a finger segment on a traditional pedicle, the frequency of venous deficiency was 34.3%, in cases of transfer of finger and metacarpal stumps—32.4%. Only one case of complete segment necrosis [n=1 (0.49%)] resulted in failure to restore hand grip, whereas all other complications were successfully managed and had no effect on the outcomes.
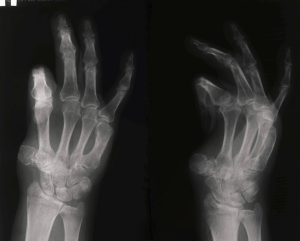
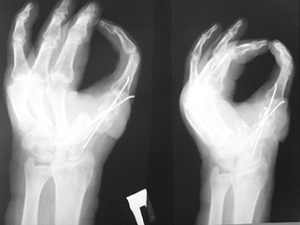
The duration of follow-up was 42±6 months. The treatment efficacy was assessed according to a modified of Belousov method (13,14). Optimal outcomes were achieved in 41.6% of cases, good outcomes—16.9% of cases, satisfactory outcomes—34.8% of cases, poor outcomes—6.7% of cases. No statistically significant difference was found in the outcomes after transferring mutilated fingers and metacarpal stumps (P>0.05). Reconstructed digits restored two-point discrimination of 6.28±0.77 mm after finger stump transfer, 7.3±0.6 mm after metacarpal stump transfer, and 3.1±0.3 mm after intact finger transfer. Poor outcomes were caused by complete defect of the I metacarpal bone and not by the nature or degree of deformity of the transferred segments or disruption of their function. Sensation in the reconstructed parts was assessed according to Weber EN (slow conduction nerve fibers) and was deemed to be satisfactory. Hand grip recovery and bony frame integrity of digits were confirmed by biomechanical, roentgenometrical, and morphological examinations. These outcomes were achieved in treating various defects of mechanical, gunshot, and thermal etiology, including cases of massive scarring and disruptions of major blood flow in segments.
It was found that mechanical ischemic training of fingers results in better blood filling and better dermal microcirculation in distal phalanx, in the I interdigital space and in the area of medial digital artery. The mean temperature increase in the distal phalanx of the bandaged finger after 10-min exposure and tourniquet removal was 6.68±1.98 °C (P<0.05) within first 2 minutes with microcirculation index increasing threefold. At peak temperature increases, we observed an increase in venous drainage and rheographical indices by 21% average (P=0.01). The identification of these trends allowed us to develop a training technique for transferred donor fingers that prevents disruptions in peripheral microcirculation in its skin cover and in the surgically formed I interdigital space [RF Patent No. 2566190 of Oct. 20, 2015, priority date Nov. 06, 2014]. The training period was 4–6 days with 4 arterial occlusions per day for 5 days: 10 min exposure on the first day, 15 min second day, 20 min third day, 25 min fourth day, 30 min from the fifth day on. The study endpoint was the difference in values of temperature increases in the distal finger segment within 3 min after tourniquet removal compared against the temperature at the end of exposure. The difference of 1.7 °C between the readings after the first and the last occlusion was considered effective, regardless of the initial condition, duration of training or number of sessions. This method was successfully used in 12 patients with severe hand damage with no prior reconstructive treatment (due to high risk of ischemic complications).
Below is a particular case demonstrating the effectiveness of the new technique.
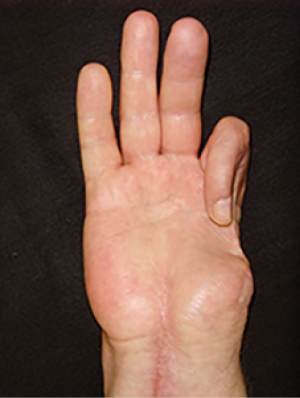
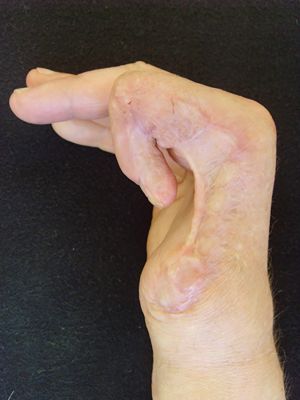
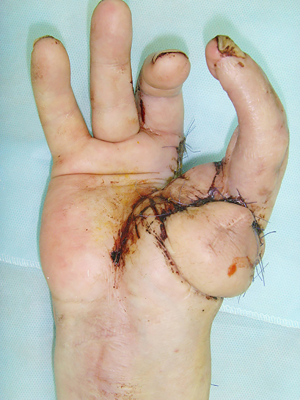
A 42-year-old man, diagnosed with total defect of radial hand side, dermato-teno-arthrogenic flexion contractures of II, III, IV fingers, soft tissues and bony frame defect, extension contracture of right hand as a result of a severe mechanical injury (Figures 2-4). In 2 years and 5 months after injury, we performed one-stage transfer of the most mutilated II finger into the thumb position on palmar and dorsal pedicles after reconstructing the finger with skin grafting and ischemic training using our method and IR observations.
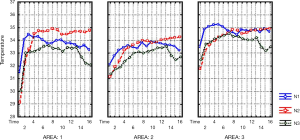
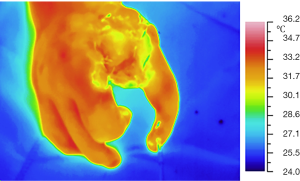
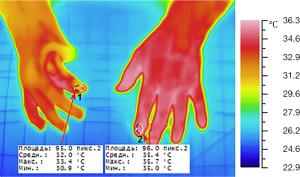
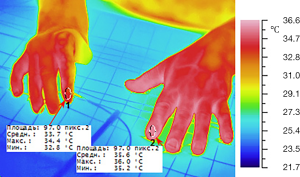
The charts (Figure 5) and thermal maps (Figures 6,7) show positive dynamics in the investigated areas at the stage of recovery after occlusion as a result of preoperative ischemic training. The transferred segment and flaps were accepted completely which allowed us to form an adequate thumb and I interdigital space (Figures 8,9). The wounds healed by primary intention healing with adequate blood supply, see Figure 10. The hand grip was restored in spite of severe initial deformity and lack of function in the donor segment (Figure 11).
Discussion
Transfer of fingers, finger or metacarpal stumps after hand injuries and their effects results in satisfactory outcomes. A drawback of this technique is narrow indications that account for lack of data on such surgeries. Many surgeons are reluctant to use mutilated fingers because of high risk of necrosis, especially in cases of finger deformations, severe scarring, and vascular damage. These complications negatively affect the function and shape of a hand that is already damaged. Besides, there is no consensus among experts whether it is appropriate to use severely deformed and often non-functional segments to reconstruct a digit and whether additional preventive measures against vascular complications are needed.
Ischemic prevention methods are mostly conservative and include drugs, physical stimuli and fixing the hand in elevated position. Surgical methods are less common and include primary arterial or venous revascularisation of the segment (1). The literature and our experience attest to the applicability of this approach in cases with intact vessels and tissues or no severe cicatricial changes, that mean that the risk and severity of ischemic complications are low (15-17). In cases of deep massive scaring such approaches are not adequate and often inefficient due to obliteration, depression and/or pathological changes in walls of arteries and veins. Even restoring major blood flow does not ensure adequate prevention against ischemic complications in scarred tissues caused by microcirculation and tissue metabolism disruptions. In such cases, an additional (dorsal) dermal pedicle can be formed using traditional technique, however, this is only appropriate in transfers of the segments adjacent to the donor stump. In cases of scarred and severely deformed segments—especially fingers and stumps at distal levels—the frequency of necrosis markedly increases (18,19), with necrosis most commonly occurring in the distal segments. The analysis of our outcomes indicates that the severity and incidence rate of such complications correlate to the scale and depth of cicatricial tissue damage, the degree of deformity and the distance of transfer.
To enhance the blood supply in non-free cutaneous-adipoid and cutaneous-muscular flaps, the Blair technique of delayed flap transfer is used (20). Our study shows that performance of tissues has proven efficient for transfers of complex scarred dermato-bony hand structures as well. The structures were trained using non-traditional techniques, including distraction. Distracted palmar and dorsal pedicles ensured adequate blood supply in transferred segments even in cases of defects, cicatricial depression, obliteration of digital arteries and veins, which is most common after burns and gunshot wounds. This effect can be explained by angiogenesis and vessel growth in distracted pedicles, which has been morphologically proven. Measured and controlled ischemic load on tissues achieved in surgical training of the transferred complex improves its resistance to hypoxia and results in metabolic adaptation, longitudinal positioning of the vessels in the pedicle and better tissue blood flow (21). As a result, the acceptability of segment tissues is enhanced. Besides, pedicles are becoming longer which allows to transfer a remotely situated metacarpal stump and any finger at proximal levels—including the procedures with two pedicles across remaining fingers or stumps—and thus perform a reconstruction of thumb and/or any finger with minimal donor defect (22).
Non-damaging selective hypoxic load on donor tissues can be affected by regular mechanical clamping of vessels of temporary pedicles in transferred tissue complexes by a constrictor or an elastic tourniquet—a technique successfully applied for peripheral revascularization of non-free groin flaps (23). This approach is not applicable when permanent pedicles are used and when triggering of other ischemic compensation mechanisms is needed. In various hand reconstruction procedures which employ its donor resources, regular preoperative tissue ischemization is performed by applying arterial tourniquet to arm or forearm. However, the length of exposures in limited by neurogenically and ischemically induced pains, that reduces the efficacy of segment training and lacks selectivity. Our approach is based on targeted ischemic preconditioning of donor segment itself. This ensures selective hypoxic load on its distal segments that are most susceptible to necrosis. The training causes minor pain that prevents angiosperms. As a result, the duration and tolerability of occlusion are increased, which in turn increases the efficacy of tissue preconditioning of the donor segment as well as the I interdigital space.
Our experience shows that, when transferring hand segments with severe and critical deformations, tissue scarring and vascular damage, it is beneficial to pre-train the donor segment against hypoxia by both exsanguination and surgical methods. This does not rule out the application of traditional techniques of transfer-segment revascularization. The results show that the developed technique allows to minimise contraindications for surgery of anatomical, morphological and functional nature on the donor segment as well as the damaged hand as a whole.
In conclusion, the use of technique described in this paper while transferring hand segments with severe and highly severe deformations, tissue scarring and vascular damage results not only in digit viability but also stability, mobility and range of motion. It also results in subjective appreciation of the patient as evidenced by actions he could not do before surgery and is possible to do after surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local ethics committee (NNIITO-2012/12/28) and all participants gave informed consent.
References
- Foucher G, Hoang PH, Dury M. La pollicisatione en urgence et en secondaire de segments digitaux mutiles. Ann Chir plast esthet 1988;33:54-7. [PubMed]
- Schoofs M, Leps P. Value of pollicization in reconstruction of the injured thumb in adults based on a series of 15 Cases. Ann Chir Main Memb Super 1992;11:19-26. [Crossref] [PubMed]
- Ishida O, Taniguchi Y, Sunagawa T. Pollicization of the index finger for traumatic thumb amputation. Plast Reconstr Surg 2006;117:909-14. [Crossref] [PubMed]
- Goldfarb CA, Monroe E, Steffen J, et al. Incidence and treatment of complications, suboptimal outcomes, and functional deficiencies after pollicization. J Hand Surg Am 2009;34:1291-7. [Crossref] [PubMed]
- Manske PR. Index pollicization for thumb deficiency. Tech Hand Up Extrem Surg 2010;14:22-32. [Crossref] [PubMed]
- Thatte MR, Nehete S, Garude K. Unfavourable results in pollicisation. Indian J Plast Surg 2013;46:303-11. [Crossref] [PubMed]
- Bravo CJ, Horton T, Moran SL, et al. Traumatized index finger pollicization for thumb reconstruction. J Hand Surg Am 2008;33:257-62. [Crossref] [PubMed]
- Gohritz A, Dellon AL, Müller FE, et al. Otto Hilgenfeldt (1900-1983): tribute to an important pioneer of European hand surgery. J Hand Surg Eur Vol 2012;37:205-10. [Crossref] [PubMed]
- Vainer BG, Markel A. Systemic vascular response to brachial arteries crossclamping remote ischemic preconditioning. Med Hypotheses 2015;84:298-300. [Crossref] [PubMed]
- Volovik MG, Kiselev DV, Polevaya SA, et al. The Effect of Repeated Local Ischemia on the Temperature and Skin Microcirculation in Human Hand. Fiziol Cheloveka 2015;41:100-9. [PubMed]
- Wretman D. Finding regions of interest in a decision support system for analysis of infrared images. Stockholm, Sweden: Master of Science Thesis, 2006:65.
- Krupatkin AI. Blood flow oscillations at a frequency of about 0.1 Hz in skin microvessels do not reflect the sympathetic regulation of their tone. Fiziol Cheloveka 2009;35:60. [PubMed]
- Belousov AE, Gubochkin NG. Complex evaluation of the results of emergency microsurgical operations in injuries of the limbs. Vestn Khir Im I I Grek 1984;132:110-3. [PubMed]
- Koziukov VG, Tokarev AE, Sevostyanov AN, et al. Ways to improve reconstructive and plastic surgery outcomes in cases of hand injury. Ural Medical Journal 2011;10:136-9.
- Chafik D, Harness NG, Lawrence JF. Unusual arterial anatomy in a case of index finger pollicization. J Hand Surg Br 2006;31:347. [Crossref] [PubMed]
- Kumar B, Acharya A, Bhat AK. A re-look at pollicization. Indian J Plast Surg 2011;44:266-75. [Crossref] [PubMed]
- Kozin SH. Pollicization: The Concept, technical details and outcome. Clin Orthop Surg 2012;4:18-35. [Crossref] [PubMed]
- Nguyen VN. Pollicization of fingers and their stump in cases of thumb loss. Sc.D. Medicine thesis. Leningrad, 1989:36.
- Dadalov MI. Reconstructive surgeries in cases of thumb loss. Ph.D. Medicine thesis. Leningrad, 1990:51.
- Blair VP. The delayed transfer of long pedicle flaps in plastic surgery. Surgery Gynec Obstet 1921;33:261.
- Pang CY, Forrest CR, Neligan PC, et al. Augmentation of blood flow in delayed random skin flaps in the pig: effect of length of delay period and angiogenesis. Plast Reconstr Surg 1986;78:68-74. [Crossref] [PubMed]
- Azolov VV, Alexandrov NM, Petrov SV. Application of Ilizarov device for transfer of deformed digits, digital stumps and metacarpal stumps. J Hand Surg Am 2009;34:1531-40. [Crossref] [PubMed]
- Furnas DW, Lamb RC, Achauer BM. A pair of fiveday flaps: Early division of distant pedicles after serial cross-clamping and observation with oximetry and fluorometry. Ann Plast Surg 1985;15:262-7. [Crossref] [PubMed]