Alcohol use disorders before and after bariatric surgery: a systematic review and meta-analysis
Introduction
Obesity is rapidly increasing in prevalence in Western society, with the Australian Health Survey (2011) revealing 9.6% of adults suffered from either class 2 (BMI, 35 kg/m2) or class 3 (BMI, 40 kg/m2) obesity (1). There is an inverse relationship between rates of obesity and socioeconomic status, with 25.3% of the population in the most disadvantaged area being class 2 or 3 obese. There is a well-documented effect of obesity on health, with impacts on both physical and psychosocial health (2). These include impaired reproductive functioning, physical limitations and impairment to quality of life. Thus, focus on treatment of obesity can significantly reduce morbidity as well as mortality.
As a reflection of the global obesity epidemic, there is an increasing number of candidates for bariatric surgery as a means of an effective and durable treatment for severe obesity (3-7). Bariatric surgery is currently indicated in class 3 obesity alone or class 2 provided there are other comorbid conditions such as sleep apnoea, diabetes, or hypertension. Bariatric surgery offers weight loss, with maintenance, and a reduction in these comorbid conditions as well as improved quality of life (3-7). There is also evidence to demonstrate its cost-effectiveness compared to conservative measures (8-10). Bariatric surgery is usually performed in one of two ways. These are the Roux-en-Y gastric bypass (RYGB) and gastric banding procedures. With RYGB procedures the weight loss is achieved by allowing food to bypass the majority of the stomach as well as some of the small intestine. In essence, a smaller stomach pouch is created. There is then a decreased transit time for food resulting in a malabsorptive state. Gastric banding, on the other hand, serves to constrict the upper stomach promoting early satiety.
Of concern, however, are the reports of increased risk of alcohol use disorder (AUD) in the post-operative period (11-13). One study conducted with the aim of observing the relation of AUD in post-bariatric surgery patients prospectively followed 1,945 patients (13). While no increase of AUD frequency was observed in the first year post-operatively, a significant increase in AUD was seen at the 2-year period when compared to the first year [frequency of 7.3% in the first year vs. 9.6% in the second (P=0.01)]. These findings were true for the patients undergoing RYGB but not gastric banding. Another study also concluded there was an increase in the frequency of AUD 2 years post bariatric surgery (14). Svensson et al. (2013), was another large study investigating AUD in post-bariatric surgery patients (n=2,010) and compared it to a non-surgical control group (n=2,037) (11). These patients were followed up to 20 years post operatively. The study concluded the patients undergoing RYGB were 3 times more at risk of developing AUD compared to the control group. It has been hypothesized that bariatric procedures have the potential to alter alcohol pharmacokinetics, reaching higher peak alcohol levels compared to patients with surgery and as such hypothetically increasing the risk of alcohol use sensitivity, predisposing these patients to AUD.
Conflicting studies do exist however with some even suggesting a decrease in alcohol consumption post-surgery. One such study involving 541 patients reported a significant reduction in AUD in patients undergoing either RYGB or gastric banding (15). There was a greater reduction in AUD in the patients undergoing gastric banding.
The aim of this study is to observe the rate of AUD in the postoperative period following bariatric surgery for weight management. To our knowledge this is the first study to conduct a meta-analysis on this. We hypothesized that patients undergoing bariatric surgery experience a raised likelihood of postoperative AUD.
Methods
The present systematic review and meta-analysis followed recommended PRISMA and international collaborative guidelines (16,17).
Literature search strategy
Electronic searches were performed using Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club, and Database of Abstracts of Review of Effectiveness (DARE) from their dates of inception to January 2017. We combined the terms: “alcohol use disorder”, “AUD”, “anxiety”, “psychosocial”, “bariatric”, “Roux-en-Y”, “banding”, “sleeve”, “gastrectomy”, as either key-words or mapped to MeSH terms. We reviewed the reference lists of all retrieved articles for further identification of potentially relevant studies, assessed using the inclusion and exclusion criteria.
Selection criteria
Eligible studies for the present systematic review and meta-analysis included those in which patient cohorts underwent a form of bariatric surgery for obesity, with the prevalence of AUD measured or reported before and after surgery. Studies that did not include the proportion of patients with AUD before and after surgery were excluded. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for quantitative assessment at each time interval. All publications were limited to those involving human subjects and in the English language. Abstracts, case reports, conference presentations, editorials, reviews and expert opinions were excluded.
Data extraction and critical appraisal
All data were extracted from article texts, tables, and figures. Two investigators independently reviewed each retrieved article (H Azam, K Phan). Discussion and consensus resolved discrepancies between the two reviewers. Because quality scoring is controversial in meta-analyses of observational studies, two reviewers (H Azam, K Phan) independently appraised each article included in our analysis according to a critical review checklist of the Dutch Cochrane Centre proposed by MOOSE (18).
Statistics
A meta-analysis comparing pre- vs. post-treatment proportion of patients with depression was conducted. For each study, pre- and post-data were compared to derive an odds ratio (OR) and confidence interval (CI), which was then pooled using random-effects model, which assumed that there were variations between studies. The results using the random-effects model were presented to take into account the possible clinical diversity and methodological variation between studies. χ2 tests were used to study heterogeneity between trials. I2 statistic was used to estimate the percentage of total variation across studies, owing to heterogeneity rather than chance, with values greater than 50% considered as substantial heterogeneity. I2 can be calculated as: I2 =100% × (Q − df)/Q, with Q defined as Cochrane’s heterogeneity statistics and df defined as the degree of freedom. Specific analyses considering confounding factors were not possible because raw data were not available. All P values were two-sided. All statistical analysis was conducted with Comprehensive Meta-Analysis (CMA version 2.2.064, Biostat, Englewood, NJ, USA).
Results
A total of 17 studies met the search criteria (Figure 1). Of these, three combined AUD and drug use disorder as one variable and were excluded. Four papers did not provide baseline alcohol use statistics and was excluded. After further evaluation of the remaining articles and assessment of the inclusion criteria, ten studies were included for the meta-analysis (Table 1). Data including bariatric surgery type, the prevalence of AUD at baseline and 1, 2 and 3 years post-surgery, as well as incidence of AUD was extracted.
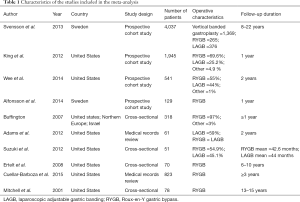
Full table
Impact of bariatric surgery on AUD prevalence at 1 year
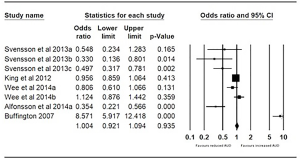
Pre- and post-bariatric surgery AUD prevalence was reported by five studies (11,13,15,19,20). Svensson et al. (11) reported three subgroups including gastric bypass, gastric banding, and vertical banded gastroplasty. King reported outcomes for all bariatric surgery, as well as the Roux-en-Y bypass, laparoscopic adjustable banding, banded gastric bypass, sleeve gastrectomy and biliopancreatic diversion. Wee et al. (15) reported outcomes for gastric bypass and gastric banding. Alfonsson et al. (19). reported outcomes for gastric bypass and Buffington (20) reported outcomes for gastric bypass predominantly. The pooled odds were 1.004 (95% CI, 0.921–1.094; P=0.935), with no significant difference found in the proportion of patients with AUD at 1 year vs. pre-surgery (Figure 2).
Impact of bariatric surgery on AUD prevalence at 2 years
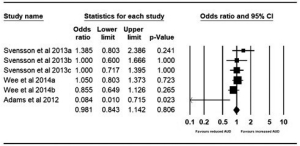
Pre- and post-bariatric surgery AUD prevalence at 2 years were reported by three studies. Adams et al. reported outcomes for gastric banding and gastric bypass pre-surgery and 2-year postoperatively (21). The pooled odds were 0.981 (95% CI, 0.843–1.142; P=0.806), with no significant difference found in the proportion of patients with AUD at 2 years vs. pre-surgery (Figure 3).
Impact of bariatric surgery on AUD prevalence at 3+ years
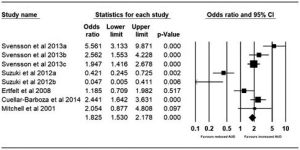
Pre- and post-bariatric surgery AUD prevalence at three years was reported by five studies. Suzuki et al. (12) reported outcomes for gastric banding and gastric bypass pre-surgery and an average of 44-month post-operative follow-up. Ertelt et al. (22) reported outcomes for gastric bypass with a follow-up range of 6–10 years postoperatively. Cuellar-Barboza et al. (23) reported outcomes for gastric bypass and Mitchell et al. (24) reported outcomes for gastric bypass up to 15 years postoperatively. The pooled odds were 1.825 (95% CI, 1.530–2.178; P<0.001) with a significant increase in AUD at the 3+ years postoperatively (Figure 4).
Risk of bias assessment
The risk of bias for each included study was assessed using the MOOSE criteria (18) (Table S1).

Full table
Discussion
Bariatric surgery is an increasingly popular and cost-effective treatment for morbid obesity, with effects being long-term while having proven benefit on the complications of obesity including hypertension, type 2 diabetes mellitus and obstructive sleep apnoea (25). Increasing reports of AUD post-surgery, however, has been concerning and this study aimed to address this question via a systematic review and meta-analysis of the available evidence. Our analysis demonstrated no significant increased prevalence of AUD from any type of bariatric surgery in the first two years of the post-operative period. However, beyond this period there is an increased risk of patients developing AUD.
Our results are corroborated by several previous studies. Svensson et al. (11) showed an increased risk of AUD in patients undergoing any bariatric surgery, with gastric bypass carrying the greatest risk. Suzuki et al. (12) demonstrated no increased risk in this period for either gastric bypass or gastric banding. The remaining three studies included for this period all favored an increased risk of AUD particularly due to gastric bypass surgery.
This increased risk of AUD was initially thought to occur due to “addiction transfer” where patients replace food consumption with alcohol consumption (26). However, this argument has been refuted as firstly it does not explain why the AUD tends to occur years after the procedure and not immediately (26)—a statement consistent with our study. Rather the effect might be due to the changed pharmacokinetics of alcohol in these patients. Observational (12) and pharmacokinetic studies (27-29) support the mechanism that alcohol sensitivity is increased following bariatric surgery, which results in higher alcohol consumption levels, particularly after the second postoperative year. This would translate into the increased prevalence of AUD.
It is known gastric bypass surgery results in reduced transit time from stomach to small bowel (30). The majority of alcohol metabolism occurs in the stomach due to the ADH enzyme, thus with the increased transit time alcohol levels in the blood increase. A study conducted to observe the effects of gastric bypass surgery on the pharmacokinetics of alcohol reported blood alcohol levels has not only a faster onset of reaching peak levels but also that the peak levels were higher in the surgical group vs. the control group matched for age and BMI (28). Interestingly, the surgical group of patients had undergone gastric bypass surgery at least three years before the study. This is consistent with our study where we report an increased risk of AUD in patients only after three years post-procedure. Patients who have undergone gastric bypass surgery also have self-reported increasing sensitivity to alcohol (18). Together these factors may explain the increasing addiction potential of alcohol in this population. Studies observing changes to alcohol metabolism in other types of bariatric surgery, namely gastric banding and sleeve gastrectomy, reported no alteration in alcohol metabolism (31,32).
The present study is constrained by several limitations. The evidence is mostly limited to cross-sectional or prevalence data, rather than time-to-event or incidence data, which limits the applicability of results. The definition of an AUD varied from study to study, and the type of bariatric procedure, surgeon skill, and volume, as well as surgical technical nuances that differ between centers could not be accounted for in our analysis. Our data were also limited to approximately 2-year follow-up so it is difficult to determine what long-term outcomes and trends would be. Long-term studies are required to determine if there is a true increase in the prevalence of AUD in the context of patients undergoing bariatric surgery procedures. Furthermore, the majority of included studies did not have a control group, and there may be a possibility that AUD in these patient groups would have increased independent of bariatric surgery.
The implications of this study suggest the need for patient counselling before gastric bypass surgery on the risk of AUD postoperatively. This is particularly important in those already with a history of AUD but also in those with no history. Patients should be screened post operatively for AUD and appropriate referrals made if issues are developing. The study also shows the need for long-term follow-up as AUD does not develop in the short-term period postoperatively, but rather after 3 years.
In conclusion prevalence of AUD increases in patients undergoing gastric bypass surgery but not gastric banding. The risk of AUD was found to not be significantly increased in the first 2 years postoperatively but increasing after that. The mechanism behind this might be due to increased sensitivity to alcohol as well as altered alcoholic metabolism following gastric bypass surgery. Implications of the study include AUD screening for a long-term period postoperatively, as well as including AUD in the counselling pre-operatively.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Australian Bureau of Statistics. Microdata: Australian Health Survey 2011-2012. Canberra. 2012. Available online: http://www.ausstats.abs.gov.au/Ausstats/subscriber.nsf/0/1680ECA402368CCFCA257AC90015AA4E/%24File/4364.0.55.001.pdf
- Sarwer DB, Lavery M, Spitzer JC. A review of the relationships between extreme obesity, quality of life, and sexual function. Obes Surg 2012;22:668-76. [Crossref] [PubMed]
- Rao RS, Kini S. Diabetic and bariatric surgery: a review of the recent trends. Surg Endosc 2012;26:893-903. [Crossref] [PubMed]
- Vest AR, Heneghan HM, Agarwal S, et al. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 2012;98:1763-77. [Crossref] [PubMed]
- Sarkhosh K, Switzer NJ, El-Hadi M, et al. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg 2013;23:414-23. [Crossref] [PubMed]
- Sjöström L, Gummesson A, Sjöström CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol 2009;10:653-62. [Crossref] [PubMed]
- Julia C, Ciangura C, Capuron L, et al. Quality of life after Roux-en-Y gastric bypass and changes in body mass index and obesity-related comorbidities. Diabetes Metab 2013;39:148-54. [Crossref] [PubMed]
- Centre for Epidemiology and Research. The health of the people of New South Wales - report of the chief health offi cer. Summary report, 2010. Sydney: NSW Department of Health, 2010. Available online: http://www0.health.nsw.gov.au/pubs/2010/pdf/chorep_summary_2010.pdf
- Dixon JB. Referral for a bariatric surgical consultation: it is time to set a standard of care. Obes Surg 2009;19:641-4. [Crossref] [PubMed]
- Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683-93. [Crossref] [PubMed]
- Svensson PA, Anveden Å, Romeo S, et al. Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study. Obesity (Silver Spring) 2013;21:2444-51. [Crossref] [PubMed]
- Suzuki J, Haimovici F, Chang G. Alcohol use disorders after bariatric surgery. Obes Surg 2012;22:201-7. [Crossref] [PubMed]
- King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA 2012;307:2516-25. [Crossref] [PubMed]
- Conason A, Teixeira J, Hsu CH, et al. Substance use following bariatric weight loss surgery. JAMA Surg 2013;148:145-50. [Crossref] [PubMed]
- Wee CC, Mukamal KJ, Huskey KW, et al. High-risk alcohol use after weight loss surgery. Surg Obes Relat Dis 2014;10:508-13. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. [Crossref] [PubMed]
- Phan K, Tian DH, Cao C, et al. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg 2015;4:112-22. [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Alfonsson S, Sundbom M, Ghaderi A. Is age a better predictor of weight loss one year after gastric bypass than symptoms of disordered eating, depression, adult ADHD and alcohol consumption? Eat Behav 2014;15:644-7. [Crossref] [PubMed]
- Buffington CK. Alcohol Use and Health Risks: Survey Results. Bariatric Times 2007;4:21.
- Adams CE, Gabriele JM, Baillie LE, et al. Tobacco use and substance use disorders as predictors of postoperative weight loss 2 years after bariatric surgery. J Behav Health Serv Res 2012;39:462-71. [Crossref] [PubMed]
- Ertelt TW, Mitchell JE, Lancaster K, et al. Alcohol abuse and dependence before and after bariatric surgery: a review of the literature and report of a new data set. Surg Obes Relat Dis 2008;4:647-50. [Crossref] [PubMed]
- Cuellar-Barboza AB, Frye MA, Grothe K, et al. Change in consumption patterns for treatment-seeking patients with alcohol use disorder post-bariatric surgery. J Psychosom Res 2015;78:199-204. [Crossref] [PubMed]
- Mitchell JE, Lancaster KL, Burgard MA, et al. Long-term follow-up of patients' status after gastric bypass. Obes Surg 2001;11:464-8. [Crossref] [PubMed]
- Edye M, Talbot ML. Inequalities of access to bariatric surgery in Australia. Med J Aust 2014;201:502-3. [Crossref] [PubMed]
- Sogg S. Alcohol misuse after bariatric surgery: epiphenomenon or "Oprah" phenomenon? Surg Obes Relat Dis 2007;3:366-8. [Crossref] [PubMed]
- Hagedorn JC, Encarnacion B, Brat GA, et al. Does gastric bypass alter alcohol metabolism? Surg Obes Relat Dis 2007;3:543-8; discussion 548. [Crossref] [PubMed]
- Klockhoff H, Näslund I, Jones AW. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. Br J Clin Pharmacol 2002;54:587-91. [Crossref] [PubMed]
- Woodard GA, Downey J, Hernandez-Boussard T, et al. Impaired alcohol metabolism after gastric bypass surgery: a case-crossover trial. J Am Coll Surg 2011;212:209-14. [Crossref] [PubMed]
- Horowitz M, Collins PJ, Harding PE, et al. Gastric emptying after gastric bypass. Int J Obes 1986;10:117-21. [PubMed]
- Gallo AS, Berducci MA, Nijhawan S, et al. Alcohol metabolism is not affected by sleeve gastrectomy. Surg Endosc 2015;29:1088-93. [Crossref] [PubMed]
- Changchien EM, Woodard GA, Hernandez-Boussard T, et al. Normal alcohol metabolism after gastric banding and sleeve gastrectomy: a case-cross-over trial. J Am Coll Surg 2012;215:475-9. [Crossref] [PubMed]