Prepsoas oblique lateral lumbar interbody fusion in deformity surgery
Introduction
The prepsoas oblique lateral lumbar interbody fusion was first described by Mayer in 1997 (1). It is an anterolateral retroperitoneal approach, which uses the corridor between the psoas and the iliac artery or aorta. In contrast to the transpoas approach, it avoids traversing the psoas muscle as well as the lumbar plexus situated within it.
Deformity correction traditionally involves extensive posterior approaches. Several techniques have been described including posterior osteotomies (2,3). These surgeries may have potentially significant surgical morbidity. Over time, minimally invasive surgeries (MIS) have been developed and are now being used in deformity cases. MIS deformity surgery has been reported to have less blood loss and lower complication rates compared to traditional open techniques. However, these techniques have been found to achieve suboptimal sagittal correction or pseudarthrosis in severe deformities (4). The minimally invasive prepsoas approach for lateral interbody fusion combined with minimally invasive pedicle screw fixation can provide good pain relief without resorting to extensive osteotomies in certain well selected cases.
Indications for prepsoas lumbar interbody fusion in deformity surgery include (5-9):
- Adult degenerative scoliosis with coronal and sagittal deformity;
- Proximal junction kyphosis;
- Post laminectomy kyphosis;
- Adjacent level disease above or below a prior fusion;
- Low grade (Meyerding grade 1 or 2) spondylolisthesis;
- Lateral listhesis;
- Pseudarthrosis.
Contraindications for this procedure include:
- High grade (Meyerding Grade 3 or 4) spondylolisthesis (May be difficult to reduce from lateral approach);
- Severe central canal stenosis;
- Severe osteoporosis;
- Prior same side retroperitoneal surgery (due to scar tissue);
- Active infection in the psoas.
Patient selection
As with any surgical procedure, patient selection is key to obtain appropriate outcomes and avoid complications. Traditional work-up includes magnetic resonance imaging (MRI) and computer tomography (CT) to identify stenosis, spondylolisthesis, and other spinal pathology. Spinal deformity patients are additionally evaluated with 36 inch long cassette X-rays to assess alignment and a variety of radiographic parameters.
Mummaneni et al. developed an algorithm to provide a framework when deciding whether a patient would be an appropriate candidate for MIS vs. open deformity correction (10). Radiographic indications for MIS/Mini-open surgery as suggested by the MISDEF algorithm include (10):
- Sagittal vertebral axis (SVA) greater than 5 cm but less than 7 cm;
- Lumbar lordosis-pelvic incidence (LL-PI) mismatch 10–30 degree;
- Lateral listhesis;
- Coronal scoliosis;
- Patients with flexible deformities that reduce to an SVA less than 6 cm on supine X-rays;
- Lack of bridging anterior osteophytes.
The following categories of patients are currently poor candidates for MIS/Mini-open techniques and are best treated with three column osteotomy or multilevel osteotomies to achieve appropriate correction. Relative radiographic contraindications for MIS/Mini-open deformity surgery are:
- SVA greater than 7 cm with a rigid curve (especially with prior instrumented fusion);
- LL-PI mismatch greater than 30 degree;
- Thoracic hyperkyphosis greater than 60 degree.
Some combined approaches can be utilized to achieve appropriate deformity correction. Lateral approaches like the prepsoas or the transpsoas can be combined with anterior longitudinal ligament release in selected cases to achieve greater lordosis.
Preoperative workup
To ensure appropriate patient selection and to assist with surgical planning, we recommend the following preoperative workup for deformity patients.
- 36 inch standing radiographs provide information about spinal alignment as well as the position of the iliac crest. A high iliac crest can make the prepsoas approach at L4–5 difficult or impossible, hence it is important to know the iliac crest position preoperatively if surgery at the L4–5 level is being contemplated. These radiographs also help in planning sagittal and coronal correction;
- Flexion extension X-rays are helpful to assess mobile spondylolisthesis;
- MRI is an essential investigation to look for spinal canal and foraminal stenosis as well as disc degeneration;
- CT scan may be ordered if detailed evaluation of specific bony anatomy is required before surgery;
- A CT angiogram is optional though usually not necessary; it will provide information about the location of aorta, inferior vena cava, iliac arteries and vein. However, most of this information can be seen on axial MRI images.
Surgical technique
Stereotactic navigation guided prepsoas oblique lateral lumbar interbody fusion
Patient positioning
The patient is positioned in the right lateral decubitus position with the left side up. Right sided prepsoas approach cannot be used because of the presence of the vena cava on the right side; this approach can only be used from the left side. A right sided approach will also place the iliac vein at risk for vascular injury. If a right sided lateral approach is mandatory for deformity correction, the transpsoas approach should probably be used instead of the prepsoas approach. It is important to ensure that the patient’s arms will not obstruct the intraoperative CT scanning.
Neuromonitoring
Motor evoked potentials as well as free running electromyography is used. Triggered EMG monitoring is used at our centre. Paralytic anaesthetic agents cannot be used if triggered EMG monitoring is employed as they can lead to false negative recordings.
Procedure
The reference array for navigation is securely placed 2 inches superolateral to the PSIS (posterior superior iliac spine). The intraoperative CT scan is brought in and the images thus obtained are registered with the navigation software. With the help of navigation, midpoint of the pathological disc space is localized on the skin from a true lateral projection (Figure 1).
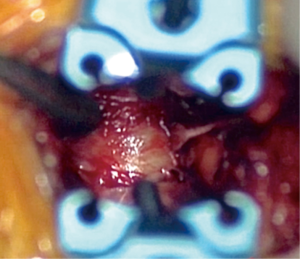
An oblique incision parallel to the abdominal wall nerve root trajectories, is made 5 cm anterior to this point. The incision is deepened to the abdominal fascia, taking care not to violate the peritoneum. The trajectory should be towards the lumbar spine and is confirmed with navigation. After careful dissection through the obliques and the transverse abdominis muscle, retroperitoneal fat is identified. The anterior border of the psoas muscle and the target disc space is confirmed with navigation and the disc space is cleared of soft tissue (Figure 2).
Sequential dilators are navigated into place with the help of triggered EMG monitoring. An incision is made at the disc annulus and a thorough discectomy is performed (Figure 3). While performing discectomy, it is important to rotate the instruments to a true lateral position before directing them towards the contralateral side. If not done, an oblique trajectory could injure the contralateral nerve root or spinal canal.
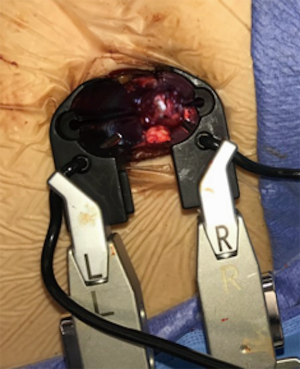
After completing the discectomy, a correct cage size is selected using interbody template trials. The implant is loaded with graft material and placed. Navigation is used to guide the trajectory and placement (Figure 4). The implant should ideally span the entire apophyseal ring in the lateral direction.
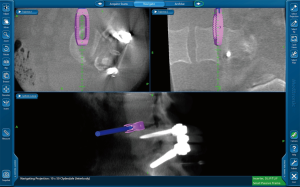
The position of the implant is routinely confirmed with fluoroscopy to ensure that the navigation was accurate during the procedure. The wound is then closed in layers.
Stereotactic navigation guided percutaneous pedicle screw insertion
Transpedicular screws are generally placed posteriorly unless anterior fixation has already been done. The posterior surgery may be staged to a different day to allow for clinical assessment between the two procedures. If radicular symptoms have resolved owing to indirect foraminal decompression, only percutaneous screws may be needed during stage 2 surgery. In case radicular symptoms persist, foraminal decompression is performed along with posterior fixation.
Positioning
The patient is positioned prone on the operating table. All bony prominences and pressure points are padded.
Neuromonitoring
Neuromonitoring with somatosensory and motor evoked potential is employed. Stimulus evoked electromyographic (EMG) monitoring may be used for detecting pedicle wall violation. Owing to the higher resistivity of the cortical bone compared to the soft tissue, EMG responses are not elicited with low stimulation electric currents if the pedicle wall is not violated. Violation of the pedicle wall allows depolarization of the adjacent nerve root as electric current flows through the low resistivity soft tissue.
Procedure
The intraoperative CT scan is brought in and the images are registered to the navigation software. The reference arc can be placed either on the spinous process or the iliac crest. The cutaneous entry point is 1–2 cm lateral to the lateral border of pedicle. The exact entry point is determined with the help of navigation; both the desired entry point in the pedicle (junction of superior and lateral margin of the pedicle) and the medial angulation is taken into account when marking the skin entry point.
After the cutaneous entry point is marked at the level of interest, a 1-cm longitudinal incision is made at this point and a navigated sharp tipped cannulated pedicle awl is advanced and placed at the junction of the lateral border of the superior facet and a bisecting line of the transverse process. The awl is advanced into the pedicle with a gentle twisting motion or with the aid of a mallet. Alternatively, a navigated cannulated drill guide and drill is used to create a tract along the pedicle (Figure 5).

A guidewire is mounted through the cannulated instruments into the pedicle. The instrument is removed once the guidewire is in place (Figure 6). It is imperative to ensure that this guidewire is not accidentally removed or displaced till the screws are in place. A small fascial incision is made to allow for screw placement over the guidewire.
The screw diameter and length is decided with the help of screw projection on navigation (Figure 7). A pedicle tap which is at least 0.5 mm smaller in diameter than the anticipated screw size is then selected. The navigated pedicle tap is then advanced over the guidewire to prepare the pedicles for screw placement. EMG stimulation can be done at this point to determine if there is a medial wall breach. The tap is then removed and cannulated screws of appropriate size are inserted with the aid of navigation. The guidewire is removed once the screw is in place. With navigation, the guidewire may or may not be used if the screw driver is navigated. The procedure is then conducted at other desired vertebral levels in a similar fashion. Once all screws are in place, the rod is advanced subfascially through the screw extensions to connect the vertebral levels.
The intraoperative CT scan is again brought in and images are obtained to confirm correct and accurate screw placement.
Postoperative management
The patient is mobilized on the 1st postoperative day. Paralytic ileus may occur, and the patient is restricted to a clear liquid diet until stools are passed. In case surgery is staged, the patient is encouraged to mobilize, to determine persistence of radicular pain after stage 1 and to decide on the need for foraminal decompression while performing posterior fixation during stage 2. Patients are discharged after they are adequately mobilizing and pain has been controlled.
Complications
The chief advantage of the prepsoas approach over the transpsoas approach is the potentially reduced incidence of lumbar plexus injury and the avoidance of surgical trauma to the psoas muscle (11,12). However, the procedure comes with its own share of potential complications.
There is a higher theoretical risk of bowel and vessel injuries due to the more anterior corridor (13). Because of the anterior corridor, there is higher theoretical risk of injury to the ureter, and it is critical to ensure that the ureter is not in the path of the dilators (14). Incisional pain and leg symptoms due to sympathetic chain injuries are common complications (15). Male sexual dysfunction has also been reported. Other reported complications include ileus, peritoneal laceration, cerebrovascular accident, lower extremity ischemia, psoas paresis, groin numbness and pseudarthrosis (13,15,16).
Clinical outcomes
Since the prepsoas oblique lateral interbody fusion was introduced, it has been increasingly used for multiple indications including adult deformity correction. Many early studies focused on the feasibility and safety of the procedure in comparison to well established procedures such as the transpoas interbody fusion or anterior interbody fusion (5,11,13,15,16). Several recent studies have investigated the both the clinical and radiographic outcomes with longer term follow-up (9,17-22). Table 1 includes recently published studies of oblique lateral interbody fusions with their reported outcomes. These studies show improvements in both patient reported outcomes such as Visual Analogue Score (VAS) back and leg pain scores, Oswestry Disability Index (ODI), and SRS-22 score, as well as improved radiographic parameters.
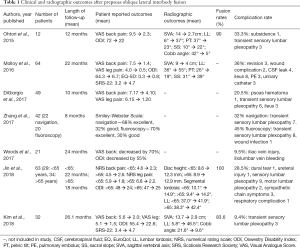
Full table
Conclusions
Prepsoas oblique lateral lumbar interbody fusion is an excellent alternative to posterior osteotomies in well selected cases as suggested by the MISDEF algorithm. Navigation can help the surgeon in performing the prepsoas lateral lumbar interbody fusion as well percutaneous pedicle screw insertion. Navigation improves the ease and accuracy of the procedure as and also decreases the radiation exposure to the surgical team (23).
Acknowledgements
None.
Footnote
Conflicts of Interest: Drs. Miller, Gulati, and Bandlish have no conflicts of interest to declare. Dr. Chou is a consultant for Medtronic and Globus and has received royalties from Globus. Dr. Mummaneni is a consultant for DePuy Spine, Globus, and Stryker, has ownership in Spinicity/ISD, and has received royalties from DePuy Spine, Thieme Publishing, and Springer Publishing and honoraria from AOSpine, as well as a grant from AOSpine and ISSG.
References
- Mayer HM. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1997;22:691-9; discussion 700. [Crossref] [PubMed]
- Bridwell KH. Decision making regarding Smith-Petersen vs. pedicle subtraction osteotomy vs. vertebral column resection for spinal deformity. Spine (Phila Pa 1976) 2006;31:S171-8. [Crossref] [PubMed]
- Suk SI, Chung ER, Kim JH, et al. Posterior vertebral column resection for severe rigid scoliosis. Spine (Phila Pa 1976) 2005;30:1682-7. [Crossref] [PubMed]
- Uribe JS, Deukmedjian AR, Mummaneni PV, et al. Complications in adult spinal deformity surgery: an analysis of minimally invasive, hybrid, and open surgical techniques. Neurosurg Focus 2014;36:E15. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Glassman SD, Hamill CL, Bridwell KH, et al. The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine (Phila Pa 1976) 2007;32:2764-70. [Crossref] [PubMed]
- Schwab FJ, Hawkinson N, Lafage V, et al. Risk factors for major peri-operative complications in adult spinal deformity surgery: a multi-center review of 953 consecutive patients. Eur Spine J 2012;21:2603-10. [Crossref] [PubMed]
- Silva FE, Lenke LG. Adult degenerative scoliosis: evaluation and management. Neurosurg Focus 2010;28:E1. [Crossref] [PubMed]
- Ohtori S, Mannoji C, Orita S, et al. Mini-Open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lateral Interbody Fusion for Degenerated Lumbar Spinal Kyphoscoliosis. Asian Spine J 2015;9:565-72. [Crossref] [PubMed]
- Mummaneni PV, Shaffrey CI, Lenke LG, et al. The minimally invasive spinal deformity surgery algorithm: a reproducible rational framework for decision making in minimally invasive spinal deformity surgery. Neurosurg Focus 2014;36:E6. [Crossref] [PubMed]
- Woods KR, Billys JB, Hynes RA. Technical description of oblique lateral interbody fusion at L1-L5 (OLIF25) and at L5-S1 (OLIF51) and evaluation of complication and fusion rates. Spine J 2017;17:545-53. [Crossref] [PubMed]
- Li JX, Phan K, Mobbs R. Oblique Lumbar Interbody Fusion: Technical Aspects, Operative Outcomes, and Complications. World Neurosurg 2017;98:113-23. [Crossref] [PubMed]
- Phan K, Maharaj M, Assem Y, et al. Review of early clinical results and complications associated with oblique lumbar interbody fusion (OLIF). J Clin Neurosci 2016;31:23-9. [Crossref] [PubMed]
- Kubota G, Orita S, Umimura T, et al. Insidious intraoperative ureteral injury as a complication in oblique lumbar interbody fusion surgery: a case report. BMC Res Notes 2017;10:193. [Crossref] [PubMed]
- Silvestre C, Mac-Thiong JM, Hilmi R, et al. Complications and Morbidities of Mini-open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lumbar Interbody Fusion in 179 Patients. Asian Spine J 2012;6:89-97. [Crossref] [PubMed]
- Patel NP, Birch BD, Dement SE, et al. The mini-open anterolateral approach for degenerative thoracolumbar disease. Clin Neurol Neurosurg 2010;112:853-7. [Crossref] [PubMed]
- Woods K, Fonseca A, Miller LE. Two-year Outcomes from a Single Surgeon's Learning Curve Experience of Oblique Lateral Interbody Fusion without Intraoperative Neuromonitoring. Cureus 2017;9:e1980. [PubMed]
- Zhang YH, White I, Potts E, et al. Comparison Perioperative Factors During Minimally Invasive Pre-Psoas Lateral Interbody Fusion of the Lumbar Spine Using Either Navigation or Conventional Fluoroscopy. Global Spine J 2017;7:657-63. [Crossref] [PubMed]
- Molloy S, Butler JS, Benton A, et al. A new extensile anterolateral retroperitoneal approach for lumbar interbody fusion from L1 to S1: a prospective series with clinical outcomes. Spine J 2016;16:786-91. [Crossref] [PubMed]
- Kim KT, Jo DJ, Lee SH, et al. Oblique retroperitoneal approach for lumbar interbody fusion from L1 to S1 in adult spinal deformity. Neurosurg Rev 2018;41:355-63. [Crossref] [PubMed]
- Jin C, Jaiswal MS, Jeun SS, et al. Outcomes of oblique lateral interbody fusion for degenerative lumbar disease in patients under or over 65 years of age. J Orthop Surg Res 2018;13:38. [Crossref] [PubMed]
- DiGiorgio AM, Edwards CS, Virk MS, et al. Stereotactic navigation for the prepsoas oblique lateral lumbar interbody fusion: technical note and case series. Neurosurg Focus 2017;43:E14. [Crossref] [PubMed]
- Villard J, Ryang YM, Demetriades AK, et al. Radiation exposure to the surgeon and the patient during posterior lumbar spinal instrumentation: a prospective randomized comparison of navigated versus non-navigated freehand techniques. Spine (Phila Pa 1976) 2014;39:1004-9. [Crossref] [PubMed]