Minimally invasive spine surgery for degenerative spine disease and deformity correction: a literature review
Introduction
Several minimal-access techniques have evolved during the last two decades in spine surgery. This evolution has been noted in endoscopic and percutaneous approaches as well as in spinal instrumentation and implants (1). The goal of minimally invasive spine surgery (MISS) is to improve clinical outcomes, reduce complications and hospital stay, and facilitate rehabilitation and return to normal activities of daily living (1). Compared to open procedures, MISS is characterized by less soft tissue damage, less blood loss, less post-operative pain, smaller and cosmetically more acceptable incisions, faster recovery, less hospital stay, and in some cases fewer complications.
Specific aspects should be taken into consideration in MISS, such as the difficulty in understanding the 3D anatomy of the spine and the difficulty in using instruments through a limited field. Another special issue with MISS is the increased cost and radiation exposure, as well as the steep learning curve (1-3).
The purpose of this review article is to discuss novel techniques of MISS for degenerative spine disease and deformity correction.
Discectomy
In the United States, lumbar disk herniation is second in incidence following upper respiratory tract infections. MISS methods have been extensively used in the surgical treatment of lumbar and cervical disc herniation during the last decade. When compared to standard microdiscectomy, minimally invasive discectomy techniques are considered less traumatic surgical procedures and have all shown promising outcomes, especially in the early postoperative period. Promising results have been reported with the tubular micro endoscopic discectomy. Another successful minimally invasive method used for the treatment of disc herniation is percutaneous endoscopic microdiscectomy technique. It is worthy to mention that in some minimally invasive discectomy procedures the patients are kept awake during the procedure enabling complete removal of the fragment (4).
In a recent meta-analysis of the literature which included 16 randomized controlled trials comparing standard discectomy versus minimally invasive discectomy for the treatment of lumbar disc herniation, it was found that minimally invasive discectomy was more likely to increase the recurrence rate and the operating time (4). On the other hand, in minimally invasive discectomy smaller incisions of the skin and fascia were used and shorter hospital stay was recorded. Another important finding was the lesser blood loss that was reported with the MISS methods and the fact that no statistically significant differences were observed in regard to the radiation exposure and cost.
Minimally invasive posterior cervical foraminotomy with or without discectomy is a well-established method for addressing cervical radiculopathy secondary to foraminal stenosis or a laterally located herniated dick. In selected patients with cervical disc herniation these MIS procedures can guarantee satisfying results in regard to pain relief and function improvement. Decreased recurrence rates and similar adjacent-level disease rates compared to standard open techniques have been reported (5).
Transforaminal lumbar interbody fusion (TLIF)
Minimally invasive transforaminal lumbar interbody fusion (MIS TLIF) was first described in 2003 (6). Optimal visualization of the operative field is possible with the introduction of special dilators and tubular retractors that reduce soft tissue damage and allows insertion of large cases. There have been several studies reporting better outcomes with MIS TLIF when compared to open TLIF in regard to length of hospital stay, blood loss, rehabilitation period, and return to activities of daily living (7,8).
MIS TLIF has been successfully used for the treatment of adult spondylolisthesis (degenerative or isthmic). MIS TLIF indications include a broad field of degenerative spine pathology, more often degenerative disc disease, disc herniation, pseudoarthrosis, and spondylosis. Patients with active infection, nerve root pathology, severe osteoporosis and extensive epidural scaring are not suitable for this technique. In patients with neurogenic claudication or radiculopathy, decompression and fusion can be performed with minimal soft tissue damage and blood loss, offering the advantage of low morbidity in elderly patients with significant co-morbidities (9). Theoretically, MIS TLIF preserves the natural posterior tension band. In addition, the use of muscle-splitting tubular retractors further limits the injury to the ipsilateral paraspinous musculature, which decreases postoperative pain.
Disadvantages include the steep learning curve, the increased operation time than conventional lumbar fusion, the difficulty to treat bilateral symptoms using a unilateral approach, and the increased radiation exposure than conventional lumbar fusion (9).
In a study by Dhall et al. (10), 21 cases of MIS TLIF were compared with 21 cases of open TLIF. Blood loss and length of hospital stay were significantly reduced in the MIS TLIF group. On the other hand, hardware complications were found to be more in the MIS TLIF group.
MIS TLIF was found superior compared to the conventional open methods in a cost-utility study (11). Less or similar complications to the open procedure were reported in several studies (12,13). According to studies reporting long-term clinical outcomes, MIS TLIF is an adequate method of treating spine pathology (14) with a steep learning curve (13).
In 318 patients who underwent MIS TLIF a fusion rate greater than 95% was reported with an average fusion time of 6.8 months (15). In another study in 64 patients, MIS TLIF was found to be superior to open TLIF in terms of Oswestry Disability Index (ODI), pain and SF-36 score improvement 2 years after surgery (8).
Lateral lumbar interbody fusion (LLIF)
The minimally invasive direct lateral retroperitoneal transpsoas approach for lumbar interbody fusion has been developed as an alternative to the well-established anterior lumbar interbody fusion (ALIF) (Figure 1). The LLIF technique combines the biomechanical and biologic benefits of ALIF when compared to dorsally-based interbody procedures with the advantages of any minimally disruptive procedure. One of the major risks of this approach is neurological injury to the lumbosacral plexus (16). In order to avoid iatrogenic complications and increase the safety of the procedures a “safe corridor” has been developed (17).
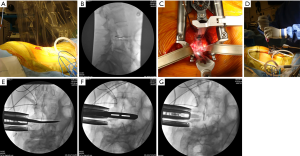
LLIF has been advocated and showed successful outcome in the setting of many adult degenerative disorders, such as degenerative disk disease and degenerative or low-grade isthmic spondylolisthesis (16-21). Degenerative pathology of the lumbar spine, such as lumbar degenerative scoliosis with laterolisthesis, can be treated with LLIF. On the other hand, extensive central canal stenosis cannot be treated with LLIF (22). Also, high grade spondylolisthesis is not suitable for LLIF. Severe facet arthropathy, deformity, abnormal vascular anatomy, previous retroperitoneal surgery and instability are contraindications for this method. Advantages of this minimal invasive procedure include improved graft-host interface, high fusion rates, decreased blood loss, early patient mobilization, and decreased hospital stay. It can provide indirect foraminal decompression and avoid direct posterior decompression.
It is also a powerful tool for the restoration of spinal alignment including correction of the coronal curve of the lumbar spine and increase of segmental lumbar lordosis in adults with degenerative scoliosis. Although no relation has been found between the development of postoperative neurologic deficit and the amount of coronal curve correction or the degree of increase in lumbar lordosis, a strong association was identified between postoperative anterior thigh/groin pain and the magnitude of curve correction or the change in lumbar lordosis (23).
Oblique lumbar interbody fusion (OLIF)
OLIF allows direct access to the disc space with preservation of the psoas muscle and without laminectomy, facetectomy or injury to the spinal or paraspinal muscles. With the patient in the lateral decubitus position, using the image intensifier the incision is made based on the disc configuration (laterally and paramedian). The levels that can be treated with OLIF are L1-S1 (22,24,25).
Almost all degenerative conditions of the lumbar spine can be treated with OLIF, especially sagittal and coronal deformity. Patients with severe spondylolisthesis and extensive canal stenosis cannot be treated with OLIF. Advantages of OLIF include fast postoperative rehabilitation, increased fusion rates and deformity correction. Vascular injury and sympathetic dysfunction have been reported following OLIF (22,26-28).
Scoliosis surgery
Adult scoliosis affects 60% of the older population and is usually asymptomatic. Symptomatic spine deformity has been reported with an incidence of 6%. Degenerative scoliosis is the result of asymmetrical disk degeneration, osteoporosis, and vertebral body compression fractures that classically present with sagittal and/or coronal imbalance, axial back pain, or radiculopathy (29). The goals in surgical treatment for spinal deformity include restoration of spinal balance, halt of deformity progression, and alleviation of radicular symptoms.
Percutaneous screws and rods insertion has the advantage of adult scoliosis correction with minimal soft tissue injury, less blood loss and faster recovery to everyday activities compared to open procedures (Figure 2) (29). Despite these advantages there are specific limitations in percutaneous scoliosis correction due to the fact that without osteotomies large coronal and/or sagittal plane deformities cannot be corrected (29). In a study by Anand et al. (30), in 71 patients successful adult spine deformity correction was reported with good to excellent functional outcomes, low pseudarthrosis rates, satisfactory clinical and radiological improvement.
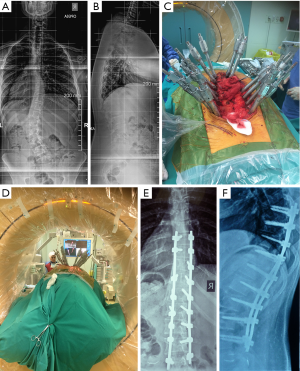
In a review of the literature, satisfactory results for 831 patients treated with MISS for adult scoliosis were reported. Coronal Cobb angle, ODI, and VAS score were significantly improved following surgery (31).
The advantage of the addition of LLIF in MISS scoliosis surgery is the decreased complication rate compared to the traditional combined open anterior and posterior procedures (22,32-34). Phillips et al. (35) reported the results in 101 patients with adult scoliosis treated with MISS, describing constant improvement in both radiological and clinical parameters at 2 years follow-up. In the same study a low complication rate was also described.
MISS through a lateral approach for the correction of adult scoliosis has been found to be a satisfactory method for both coronal and sagittal realignment of the spine (36). However, a subsidence rate of 29% has been reported, questioning the issue of further supplementation.
Discussion
During the last two decades, there has been a continuous evolution of MISS (37). However, there is still no consensus in regard to the benefits of MISS in everyday spine surgery taking under consideration the reduced soft tissue injury and faster return to everyday activities versus the stiff learning curve and the financial burden of the heavy instrumentation together with patient safety and cost-effectiveness (7,37).
A difficult learning curve and higher rates of neurological complications have been reported with MISS, setting an important issue in the debate between conventional open methods and MISS (38). On the other hand, there is data published that supports the reduced blood loss in MISS (39).
In a meta-analysis, Kamper et al. (40) did not report significant differences in clinical outcomes between open and MISS procedures. According to the same meta-analysis, many studies support decreased operating time, reduced blood loss, decreased length of hospital stay, and decreased complication or reoperation rates in MISS. In addition, reduced perioperative analgesia has been reported in MISS (41).
The steep learning curve of MISS is an issue that has to be taken under consideration. Due to the technical difficulties during the first procedures and in order to minimize the risk for intra- and post-operative complications spine surgeons should participate in organized cadaveric courses and follow important technical steps. In an effort to improve surgical skills in MISS the training period is likely to be extended and the open conventional methods are likely to be cut back (42).
Another issue with minimal invasive procedures is radiation exposure of the surgeon, the patient and the operating room stuff. According to published data radiation exposure is increased in MISS and careful pre-operative planning should be made to avoid unnecessary radiation exposure (43).
Compared to fluoroscopic guidance, spine navigation increases the accuracy of MIS screw placement to more than 97% or even to 100% when a final 3D scan is performed, which in turns, decreases the neurological complication rate, allows insertion of screws of larger diameter which increases the biomechanical strength of the whole construct and decreases the rate of implant failure or pseudarthrosis. At the same time, spine navigation decreases operative time especially in multilevel surgery, minimizes the radiation to the surgical team since none of the operating room stuff stays in the operating room when the scanning is performed, and finally with the use of the new radiation dose protocols may decrease patient radiation exposure (44).
Cost-effectiveness of MISS procedures should be taken seriously under consideration. Newer technology and instrumentation in spine surgery, computer assisted navigation and more recently robotics may increase the overall surgery cost (45,46). On the other hand, there are several studies reporting reduced overall cost with MISS (47,48) emphasizing the need for more Level I studies that encounter cost-utility analysis in order to draw safe conclusions.
Conclusions
MISS has several advantages compared to conventional open methods in selected patients. Smaller incisions and less soft tissue injury, shorter hospital stay, less blood loss and faster return to previous activities are in favor of MISS. With the use of spine navigation, robotics and cadaveric courses, the obstacles of prolonged operating time and steep learning curve can be overwhelmed for more promising outcomes with MISS. Further randomized controlled trials are still needed in order to draw clear conclusions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Banczerowski P, Czigléczki G, Papp Z, et al. Minimally invasive spine surgery: systematic review Neurosurg Rev 2015;38:11-26; discussion 26. [Crossref] [PubMed]
- Perez-Cruet MJ, Fessler RG, Perin NI. Review: complications of minimally invasive spinal surgery. Neurosurgery 2002;51:S26-S36. [Crossref] [PubMed]
- Sclafani JA, Kim CW., Foley KT, et al. Complications associated with the initial learning curve of minimally invasive spine surgery: a systematic review Clin Orthop Relat Res 2014;472:1711-7. [Crossref] [PubMed]
- Chang X, Chen B, Li HY, et al. The safety and efficacy of minimally invasive discectomy: a meta-analysis of prospective randomised controlled trials. Int Orthop 2014;38:1225-34. [Crossref] [PubMed]
- Skovrlj B, Gologorsky Y, Haque R, et al. Complications, outcomes, and need for fusion after minimally invasive posterior cervical foraminotomy and microdiscectomy. Spine J 2014;14:2405-11. [Crossref] [PubMed]
- Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine (Phila Pa 1976) 2003;28:S26-35. [Crossref] [PubMed]
- Villavicencio AT, Burneikiene S, Roeca CM, et al. Minimally invasive versus open transforaminal lumbar interbody fusion. Surg Neurol Int 2010;1:12.
- Djurasovic M, Rouben DP, Glassman SD, et al. Clinical outcomes of minimally invasive versus open single level TLIF: A propensity matched cohort study. Am J Orthop (Belle Mead NJ) 2016;45:E77-82. [PubMed]
- Rouben D, Casnellie M, Ferguson M. Long-term durability of minimal invasive posterior transforaminal lumbar interbody fusion: a clinical and radiographic follow-up. J Spinal Disord Tech 2011;24:288-96. [Crossref] [PubMed]
- Dhall SS, Wang MY, Mummaneni PV. Clinical and radiographic comparison of mini-open transforaminal lumbar interbody fusion with open transforaminal lumbar interbody fusion in 42 patients with long-term follow-up. J Neurosurg Spine 2008;9:560-5. [Crossref] [PubMed]
- Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion (TLIF) for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg 2014;82:230-8. [Crossref] [PubMed]
- Lawton CD, Smith ZA, Barnawi A, et al. The surgical technique of minimally invasive transforaminal lumbar interbody fusion. J Neurosurg Sci 2011;55:259-64. [PubMed]
- Lee P, Fessler RG. Perioperative and postoperative complications of single-level minimally invasive transforaminal lumbar interbody fusion in elderly adults. J Clin Neurosci 2012;19:111-4. [Crossref] [PubMed]
- Kim JS, Jung B, Lee SH. Instrumented minimally invasive spinal-transforaminal lumbar interbody fusion (MIS-TLIF); minimum 5-years follow-up with clinical and radiologic outcomes. J Spinal Disord Tech 2012. [Epub ahead of print]. [Crossref] [PubMed]
- Perez-Cruet MJ, Hussain NS, White GZ, et al. Quality-of-life outcomes with minimally invasive transforaminal lumbar interbody fusion based on long-term analysis of 304 consecutive patients. Spine (Phila Pa 1976) 2014;39:E191-8. [Crossref] [PubMed]
- Lykissas MG, Aichmair A, Hughes AP, et al. Nerve injury after lateral lumbar interbody fusion: a review of 919 treated levels with identification of risk factors. Spine J 2014;14:749-58. [Crossref] [PubMed]
- Regev GJ, Chen L, Dhawan M, et al. Morphometric analysis of the ventral nerve roots and retroperitoneal vessels with respect to the minimally invasive lateral approach in normal and deformed spines. Spine (Phila Pa 1976) 2009;34:1330-5. [Crossref] [PubMed]
- Arnold PM, Anderson KK, McGuire RA Jr. The lateral transpsoas approach to the lumbar and thoracic spine: a review far lateral approaches (XLIF) in adult scoliosis. Surg Neurol Int 2012;3:S198-215. [Crossref] [PubMed]
- Marchi L, Oliveira L, Amaral R, et al. Lateral interbody fusion for treatment of discogenic low back pain: minimally invasive surgical techniques. Adv Orthop 2012;2012:282068. [PubMed]
- Graham RB, Wong AP, Liu JC. Minimally invasive lateral transpsoas approach to the lumbar spine: Pitfalls and complication avoidance. Neurosurg Clin N Am 2014;25:219-31. [Crossref] [PubMed]
- Youssef JA, McAfee PC, Patty CA, et al. Minimally invasive surgery: lateral Neurosurg Rev, approach interbody fusion: results and review. Spine (Phila Pa 1976) 2010;35:S302-11. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF J Spine Surg. 2015;1:2-18. [PubMed]
- Lykissas MG, Cho W, Aichmair A, et al. Is there any relation between the amount of curve correction and postoperative neurological deficit or pain in patients undergoing stand-alone lateral lumbar interbody fusion? Spine (Phila Pa 1976) 2013;38:1656-62. [Crossref] [PubMed]
- Mayer HM. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1997;22:691-9; discussion 700. [Crossref] [PubMed]
- Silvestre C, Mac-Thiong JM, Hilmi R, et al. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J 2012;6:89-97. [Crossref] [PubMed]
- Ohtori S, Orita S, Yamauchi K, et al. Mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for lumbar spinal degeneration disease. Yonsei Med J 2015;56:1051-9. [Crossref] [PubMed]
- Ohtori S, Mannoji C, Orita S, et al. Mini-Open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lateral Interbody Fusion for Degenerated Lumbar Spinal Kyphoscoliosis. Asian Spine J 2015;9:565-72. [Crossref] [PubMed]
- Phan K, Mobbs RJ. Oblique lumbar interbody fusion for revision of non-union following prior posterior surgery: a case report. Orthop Surg 2015;7:364-7. [Crossref] [PubMed]
- Anand N, Baron EM, Kahwaty S. Evidence basis/outcomes in minimally invasive spinal scoliosis surgery. Neurosurg Clin N Am 2014;25:361-75. [Crossref] [PubMed]
- Anand N, Baron EM, Khandehroo B, et al. Long-term 2- to 5-year clinical and functional outcomes of minimally invasive surgery for adult scoliosis Spine (Phila Pa 1976) 2013;38:1566-75. [Crossref] [PubMed]
- Liu G, Liu S, Zuo YZ, et al. Recent advances in technique and clinical outcomes of minimally invasive spine surgery in adult scoliosis. Chin Med J (Engl) 2017;130:2608-15. [Crossref] [PubMed]
- Caputo AM, Michael KW, Chapman TM Jr, et al. Clinical outcomes of extreme lateral interbody fusion in the treatment of adult degenerative scoliosis. ScientificWorldJournal 2012;2012:680643. [PubMed]
- Isaacs RE, Hyde J, Goodrich JA, et al. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine (Phila Pa 1976) 2010;35:S322-30. [Crossref] [PubMed]
- Pimenta L, Lhamby J, Gharzedine I, et al. XLIF approach for the treatment of adult scoliosis: 2-Year follow-up. Spine J 2007;7:52S-53S. [Crossref]
- Phillips FM, Isaacs RE, Rodgers WB, et al. Adult degenerative scoliosis treated with XLIF: clinical and radiographical results of a prospective multicenter study with 24-month follow-up. Spine (Phila Pa 1976) 2013;38:1853-61. [Crossref] [PubMed]
- Castro C, Oliveira L, Amaral R, et al. Is the lateral transpsoas approach feasible for the treatment of adult degenerative scoliosis? Clin Orthop Relat Res 2014;472:1776-83. [Crossref] [PubMed]
- Payer M. Minimally invasive lumbar spine surgery: a critical review. Acta Neurochir (Wien) 2011;153:1455-9. [Crossref] [PubMed]
- Schizas C, Tzinieris N, Tsiridis E, et al. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Orthop 2009;33:1683-8. [Crossref] [PubMed]
- Peng CW, Yue WM, Poh SY, et al. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine (Phila Pa 1976) 2009;34:1385-9. [Crossref] [PubMed]
- Kamper SJ, Ostelo RW, Rubinstein SM, et al. Minimally invasive surgery for lumbar disc herniation: a systematic review and meta-analysis Eur Spine J 2014;23:1021-43. [PubMed]
- Harrington JF, French P. Open versus minimally invasive lumbar microdiscectomy: comparison of operative times, length of hospital stay, narcotic use and complications. Minim Invasive Neurosurg 2008;51:30-5. [Crossref] [PubMed]
- Chung RS, Ahmed N. The impact of minimally invasive surgery on residents" open operative experience: analysis of two decades of national data. Ann Surg 2010;251:205-12. [Crossref] [PubMed]
- Yu E, Khan SN. Does less invasive spine surgery result in increased radiation exposure? A systematic review. Clin Orthop Relat Res 2014;472:1738-48. [Crossref] [PubMed]
- Kassis SZ, Abukwedar LK, Msaddi AK, et al. Combining pedicle screw stimulation with spinal navigation, a protocol to maximize the safety of neural elements and minimize radiation exposure in thoracolumbar spine instrumentation. Eur Spine J 2016;25:1724-8. [Crossref] [PubMed]
- Hu X, Ohnmeiss DD, Lieberman IH. Robotic-assisted pedicle screw placement: lessons learned from the first 102 patients. Eur Spine J 2013;22:661-6. [Crossref] [PubMed]
- Smith HE, Rihn JA, Brodke DS, et al. Spine care: evaluation of the efficacy and cost of emerging technology. Am J Med Qual 2009;24:25S-31S. [Crossref] [PubMed]
- Al-Khouja LT, Baron EM, Johnson JP, et al. Cost-effectiveness analysis in minimally invasive spine surgery. Neurosurg Focus 2014;36:E4. [Crossref] [PubMed]
- Vertuani S, Nilsson J, Borgman B, et al. A cost-effectiveness analysis of minimally invasive versus open surgery techniques for lumbar spinal fusion in Italy and the United Kingdom. Value Health 2015;18:810-6. [Crossref] [PubMed]


