Improving CT screening for lung cancer with a highly predictive risk model
In 2011, the National Lung Screening Trial (NLST) found that low-dose CT screening in high-risk individuals reduced lung cancer mortality by 20% if compared to chest X-ray (1).
Since the publication of the NLST results, many medical organizations have recommended low-dose CT lung screening, based on NLST eligibility criteria or similar: age 55–74 years, a 30+ pack-year smoking history and current smoking status or having quit in the last 15 years.
Patient selection is one of the key points for an effective screening program since it remarkably influences the harms and benefits of screening and its cost-effectiveness. Unfortunately, to date, there is no common strategy for patient selection in CT screening (2). The NLST entry criteria do not quantify individual risk and a non-negligible proportion of Americans diagnosed with lung cancer fail to meet them (3).
The introduction of risk models into screening programs could have great benefit. In particular, three studies recently demonstrated that selection of individuals for lung cancer screening using accurate risk prediction models is superior to using NLST/USPSTF criteria (4-6).
In 2007, the Pan-Canadian Early Detection of Lung Cancer (PanCan) trial was designed to recruit individuals for lung cancer screening using a prototype of the PLCOm2012 risk model (7). Risk factors integrated as predictor variables to provide an individual risk stratification were: education level, family history of lung cancer, body mass index, the results of any chest X-ray performed within 3 years and presence of respiratory diseases (Table 1). The relevance of respiratory diseases for selection of high-risk subjects was confirmed by the literature (8,9). In particular, Wille et al. (10) recently reported a two-to-six fold increase in lung cancer risk in association with COPD and more than 35 pack-year, suggesting a potential beneficial effect of screening for this particular subgroup.
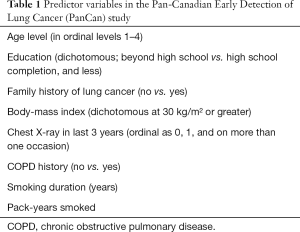
Full table
The study recently published in The Lancet Oncology by Tammemagi et al. (11) will have a significant impact on selection of high-risk subjects for lung cancer screening. In 2008–2010, they enrolled 2,537 eligible current and former smokers between 50 and 75 years, without a self-reported history of lung cancer, from eight centers across Canada. Enrolled participants must have a 6-year risk of lung cancer of at least 2%, as determined by the PanCan risk model. Patients were screened with low-dose CT scans at baseline (T0), at 1 year (T1) and finally at 4 years (T4). Median follow-up was 5.5 years; a long follow-up was crucial to assess the sensitivity and calibration of a model in which the predicted lung cancer risk is estimated for 5 or 6 years.
The primary outcome of the study was lung cancer cumulative incidence, while the secondary outcome was stage distribution of lung cancers by comparison with NLST.
One-hundred-sixty-four participants were diagnosed with lung cancer (172 total number of lung cancers), for a cumulative incidence of 6.5% (164/2,537) and an incidence rate of 138 per 10,000 person-years. The incidence was significantly greater than that observed in NLST (4%, 1,060/26,723, Table 2).
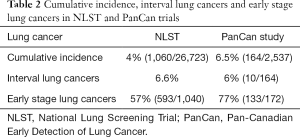
Full table
Out of the 172 lung cancers detected, 137 (80%) were identified at T0, 8 (5%) were identified at T1 and T2, 17 (10%) at T4; ten were interval cancers. The proportion of interval lung cancers (6%) was lower than NLST (6.6%) (12,13). The proportion of lung cancers that were early stage was significantly higher in the PanCan study (77%) than in NLST (57%, 593/1,040) (12).
This was the first trial that prospectively recruit individuals for lung cancer screening using a predictive risk model, based on the evaluation of risk factors beyond age a smoking history.
The only other prospective study that used a risk prediction model to select candidates was the UK Lung Screening (UKLS) trial, which enrolled individuals with a 5-year lung cancer risk of at least 5% (14). After the baseline and 12-month CT, 42 (2.1%) of the 1,994 screened participants were confirmed to have lung cancer.
The PanCan study has several limitations. First, it is not a randomized controlled trial that compared risk-model-based enrolment to NLST eligibility criteria. Another limitation was the non-negligible portion of enrolled patients lost at follow-up (752/2,537, 30%).
The results from the PanCan study are a significant step forward for lung cancer screening trials, with significant health implications (15). It remains to be seen if the high cancer detection rate, early stage shift, and relatively low incidence of interval lung cancers seen in the PanCan Study will lead to greater mortality reduction, which is the primary endpoint of a screening test. However, the PanCan results are encouraging and a recent study of Cheung et al. (16) suggest that lung cancer screening based on individual risk has the potential to save more lives than current recommendations based on NSLT eligibility criteria.
In the era of personalized medicine where we effort to tailor the therapeutic and diagnostic procedures to the needs of the individual patient, the adoption of risk models for patients selection could significantly improve early detection of lung cancers in asymptomatic heavy smokers.
Waiting for the introduction of molecular biomarkers as a potential tool for a more tailored selection of high-risk subjects, the PanCan risk model should be considered for adoption in lung cancer screening programs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Silva M, Pastorino U, Sverzellati N. Lung cancer screening with low-dose CT in Europe: strength and weakness of diverse independent screening trials. Clin Radiol 2017;72:389-400. [Crossref] [PubMed]
- Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered? J Med Screen 2012;19:154-6. [Crossref] [PubMed]
- Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, Chaturvedi AK, Silvestri GA, Riley TL, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013;368:728-36. [Crossref] [PubMed]
- Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med 2013;369:245-54. [Crossref] [PubMed]
- Tammemägi MC, Church TR, Hocking WG, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med 2014;11:e1001764. [Crossref] [PubMed]
- Tammemagi MC, Freedman MT, Pinsky PF, et al. Prediction of true positive lung cancers in individuals with abnormal suspicious chest radiographs: a prostate, lung, colorectal, and ovarian cancer screening trial study. J Thorac Oncol 2009;4:710-21. [Crossref] [PubMed]
- Brenner DR, Boffetta P, Duell EJ, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am J Epidemiol 2012;176:573-85. [Crossref] [PubMed]
- Calabrò E, Randi G, La Vecchia C, et al. Lung function predicts lung cancer risk in smokers: a tool for targeting screening programmes. Eur Respir J 2010;35:146-51. [Crossref] [PubMed]
- Wille MM, Dirksen A, Ashraf H, et al. Results of the Randomized Danish Lung Cancer Screening Trial with Focus on High-Risk Profiling. Am J Respir Crit Care Med 2016;193:542-51. [Crossref] [PubMed]
- Tammemagi MC, Schmidt H, Martel S, et al. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol 2017;18:1523-31. [Crossref] [PubMed]
- Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920-31. [Crossref] [PubMed]
- Patz EF Jr, Greco E, Gatsonis C, et al. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncol 2016;17:590-9. [Crossref] [PubMed]
- Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. [Crossref] [PubMed]
- Cressman S, Peacock SJ, Tammemägi MC, et al. The Cost-Effectiveness of High-Risk Lung Cancer Screening and Drivers of Program Efficiency. J Thorac Oncol 2017;12:1210-22. [Crossref] [PubMed]
- Cheung LC, Katki HA, Chaturvedi AK, et al. Preventing Lung Cancer Mortality by Computed Tomography Screening: The Effect of Risk-Based Versus U.S. Preventive Services Task Force Eligibility Criteria, 2005-2015. Ann Intern Med 2018;168:229-32. [Crossref] [PubMed]


