Surgical approach in the oligometastatic patient
Introduction
Metastatic patients are usually deemed to be incurable and no local aggressive radical treatments are generally indicated, instead of mere palliative support in order to maintain their quality of life. Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related mortality. As reported by the International Agency for Research on Cancer EUCAN (1), almost three-hundred thousand new NSCLC cases were diagnosed in Europe in 2012 with a cumulative overall mortality rate of 59.1%, as the majority of patients were diagnosed with an advanced stage disease. Stage IV NSCLC usually presents a dismal prognosis (2) due to an unpredictable pattern of spread (3) with both multiple and solitary metastases (4), which results in poor median survivals of 8–11 months and a 5-year overall survival (OS) of only 4–6% (5,6). For these reasons, the rationale for metastatic non-small cell cancer relies only in chemotherapy as systemic treatment associated with palliative strategies for symptoms release and optimization of quality of life (7-10). Surgery is rarely indicated though encouraging reports of long-term survival patients with low-volume stage IV disease treated with local aggressive protocols have emerged. Moreover, reticence to surgical approaches is supported by recent remarkable advances in chemotherapy strategies or immunotherapy concerning with survival benefits (11), especially for patients presenting genic mutations or re-arrangements (12). Yang et al. (13), in a recent phase III trial evaluating target and cisplatin-based regimens, reported significant survival benefits in 19delEGFR-stage IV NSCLC patients (median overall-survival: 33.3 vs. 21.1 months). These evidences have certainly led some to reconsider the role of surgical resection for patients with NSCLC and to move up criticisms towards suspects of bias in the studies involved. In this setting, the undisputed role of surgery has significantly decreased, although currently it is newly gaining place in management of stage IV NSCLC in patients with intrathoracic local control disease progression (14). Moreover, it is strongly clear that the stage IV NSCLC is a heterogeneous group as confirmed also by the eight TNM NSCLC staging system. In fact, a reclassification of metastatic disease has been accomplished into three cohorts (M1a-c) according to site and number of metastases (15,16) and reflecting a significant difference in median survival (M1a 22.5 months, M1b 17.8 months and M1c 13.6 months, P<0.001) (17).
Oligometastatic disease: state of art
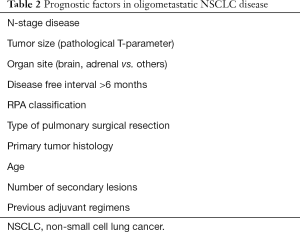
Oligometastatic disease is widely recognized as patients with a limited number and controllable secondary lesions (18), whose actual incidence in NSCLC relies between 2% and 7% according different experiences (19,20). However, the definition of oligometastases is still debated due to the presumption of a subclassification drift in the absence of real clinical findings. In a recent study by Griffioen et al. (21), involving 61 retrospectively reviewed M1b-oligometastatic NSCLC patients, many aspects concerning both number and site of metastases have been clarified. In fact, the authors identified an ideal cut-off of up to three metastases in which radical treatment seemed to provide a survival benefit associated with a significant prognostic difference concerning metastatic site (i.e., negative factor in bone lesions). Another crucial aspect to consider, is the distinction between an oligometastatic state at diagnosis and oligorecurrence. This latter, as proposed by Niibe et al. (22), should be intended as the appearance of up to five secondary lesions amenable to local aggressive treatment in a patient with a controlled primary lung cancer. From a clinical point of view, oligometastases account different scenarios from oligometastatic disease at diagnosis, oligorecurrence, oligoprogression during an adjuvant regiment due to genic mutations (23) and patients with residual oligometastases after chemoradiotherapic regimens reflecting different mRNA patterns and not the outcome of a M1c-stage disease (24) (Table 1). Moreover, much of evidences come from surgical reports without any level 1 evidence support from trials which could lead to misinterpretations or overestimation of results leading to bias (25). Concerning with site of dissemination, Ashwort et al. (26), in a fascinating review involving 49 publications and 2,176 NSCLC patients, reported brain secondary lesions are the most common (60.3%) followed by multiorgan metastases such as contralateral lungs, nodes, liver (23.0%) and adrenal gland ones (10.6%). Notwithstanding the high propensity for metastatization and rapid progression, most of patients (85%) presented a type I oligometastatic disease. However, data claim criticisms about selection bias presenting almost more than half of patients a primary lung adenocarcinoma, whose propensity to M-disease is significantly inferior to other histotypes. In regards to prognosis and factors affecting both progression free survival (PFS) and OS, M1-oligometastatic disease is influenced by many factors though just only local aggressive treatments seem to be associated to a conspicuous and significant increase in OS (median survival 19 vs. 14.8 months) (26) (Table 2). Other prognostic factors are: N-stage disease, tumor size, organ site, disease free-interval greater than 6 months, RPA classification, the presence of extracranial metastases, type of pulmonary surgical resection, primary tumor histology, age, number of secondary lesions, previous adjuvant regimens. Concerning with N-stage, Tamura et al. (27), in a retrospective cohort study involving 761 stage IV NSCLC patients, reported N0 patients presented a significant increased survival when compared to N1–3 ones (11.9 vs. 7.2 months, P<0.001), thus suggesting lymph node metastases were independent prognostic factors. Similarly, tumor size seems to strongly influence prognosis, as reported by Collaud et al. (28) in a small retrospective single-centre study. The authors, involving 29 surgically treated oligometastatic patients, showed a 1- and 5-year OS of 65% and 36%, respectively. Moreover, patients with low pathological T-stage (T1–2) gained survival when compared with advanced ones (T3–4). Hanagiri et al. (3), in a study involving 36 NSCLC patients, highlighted patients with type I metastatic disease presented a 5-year OS of 50.3%, while patient with more than two distant metastases has a 5-year OS of only 16.7%. Finally, when consider prognosis in stage IV disease, another significant aspect to consider is the site of metastases. In particular, brain or adrenal gland secondary lesion seem to be associated with prolonged survival rather than bone or liver ones (21,29-35). Referring to the above risk factors, it is possible to stratify and identify patients who are candidates for aggressive local treatment. In this setting, surgical metastasectomy is the most common strategy for local control of an advanced disease amenable to R0 resection. However, less invasive techniques have gained consensus in recent years, such as stereotactic radiosurgery (SRS), firstly for cerebral metastases and then extended to extracranial sites such as adrenal glands [stereotactic ablative radiotherapy (SART)] and lungs or liver [stereotactic body radiation radiotherapy (SBRT)]. However, these strategies are not intended as substitutes for surgery but an aid and a resource that can be spent in patients unfit for demolitive procedures, ensuring excellent local control with reported 2-year rates up to 90% (36). On the other side, outcomes concerning quality of life and long term sequelae are still controversial with discordant adverse events (toxicity) rates up to 33% and with a 30-day mortality of 7% (26).

Full table
Full table
Secondary lesions according to site in oligometastatic NSCLC
Brain metastases
Secondary lesions to the nervous system are common in cancer patients (37) and occurs up to 25% of NSCLC patients (38-40), as brain metastases are diagnosed as the first site of recurrence in cancer patients. Moreover, brain lesions are likely to increase as the results of new systemic and target protocols with concurrent long-survivor patients (41). According to the American College of Chest Physicians (ACCP) Guidelines (42), an oligometastatic brain NSCLC should be careful investigated since the limitation to the radical treatment of metastasis is the control of the primary disease. For these reasons, invasive and non-invasive mediastinal staging as far as extrathoracic imaging are strongly suggested. In fact, local aggressive strategies, such as surgery or radiotherapy, are recommended only in absence of a N2 disease and in the setting of a controlled primary lung cancer (Table 3). Radiotherapy, as fractionated whole brain irradiation therapy (WBRT) or intracranial radiosurgery (SRS), plays a critical role in the treatment of oligometastatic brain NSCLC patients (43,44) and, in particular, SRS seem to afford an improved local tumor control as well as neurocognitive function preservation (45). Chang et al. (46), in a randomized controlled trial involving 58 patients, reported a high probability (96%) that patients randomly assigned for SRS + WBRT were significantly more likely to show a decline functions, when compared to SRS brace. In particular WBRT sequelae and declines in quality of life (QoL) are variable and comprising states from moderate to severe dementia and memory reduction (47,48). For these reasons, radiosurgery seems to have overcome classical regimens, as reported by a recent retrospective study from the United States National Cancer Database (NCDB) with a current SRS-protocol rate of about 12% (49). Concerning with prognostic factors for brain metastatic disease, many scores have been proposed although the recursive partitioning analysis (RPA) classification system is the most accepted (50). It presents three prognostic classes according to age, control of primary tumor, Karnofsky Performance Status and the presence of extracranial disease. On the other hand Sperduto et al. (51) introduced, in a review from the RTOG database involving 1,960 patients, a graded prognostic assessment score (GPA). However, lack of consensus still remains and especially in the light of QUARTZ trial results (52). Nodal involvement (N0 vs. N+ disease) is another factor to consider. Arietta et al. (53), reporting thirty M1b-brain NSCLC patients treated with WBRT, showed a DFI and a OS of 8.4 and 31.8 months respectively with a 1- and 2-year OS rates of 71.1% and 60.2%. Among the prognostic factors, the Authors demonstrated the prognostic value of the nodal involvement (N0 vs. N+: 60% vs. 24%; P=0.038). Concerning with performance status and local status, Flannery et al. (54) reported 42 patients with synchronous SRS-treated single brain metastases with a 1-, 2- and 5-year OS of 71.3%, 34.1% and 21%. At the multivariate analysis definitive thoracic therapy (i.e., local tumor control) and Karnofski Performance Status were the only significant prognostic factors (P=0.020 and P=0.001, respectively). Similar results were presented by Hu et al. (55), in a retrospective study involving 84 M1b-brain synchronous metastases treated both with SRS and surgery. The median OS according to TN parameters were significantly different (stage I to III: 25.6 vs. 9.5 and 9.9 months, P=0.006). Other prognostic factors are age (19), CEA levels (56) and histology (primary pulmonary adenocarcinoma) (54). This latter aspect leads criticisms due to inhomogeneous and sometimes disagreeable results. In fact, Bella et al. (57), in a retrospective study involving 645 NSCLC patients and 25 of those with M1-brain disease, reported no significant differences in prognosis when histotype is considered (P=0.57). In regards with surgery, ESMO guidelines (58) suggest that the aggressive local ablative strategies with SRS should be reserved only for patients with RPA class I or II up to three metastases and with SRS or surgery in presence of a single metastasis (Table 3). On the other hand, no aggressive treatment should be offered for class III patients. Historically, early cohort studies involving NSCLC patients with oligometastatic central nervous secondary lesions extensively described locally aggressive surgical strategies with very variable survival rates (59). However, later on, the efficacy of stereotactic protocols or other ablative techniques took hold leading to an extensive restaging of M1 disease according to subgroups fit to different strategies, as also reported by the ESMO guidelines (58). In a retrospective study involving 12 from 170 NSCLC patients who underwent curative surgical brain metastasectomy, Daniels et al. (33) highlighted an excellent 5-year OS of 70%. Synchronous radically-treated brain oligometastases present an encouraging median survival up to 65months and a 1-year OS up to 95%. Survival rates are, otherwise, strongly influenced by pulmonary control (53). Otherwise, Bae et al. (60), in a cohort of 86 metachronous brain M+ NSCLC patients, underlined a 5-year OS of 22% suggesting that delayed disease-relapse seems to hesitate in a worse prognosis. However, results seem to be conflicting. Bonnette et al. (61), in a multicenter retrospective study involving 103 synchronous brain metastatic patients, reported a 2- and 5-year OS of 28% and 11%. Mordant et al. (19), in a national study involving 4,668 NSCLC patient and 57 brain stage IV disease, displayed a dismal prognosis of only 13%. Wroński et al. (62), in an historical single-centre retrospective study involving a significant number of patients with brain metastases (n=231), showed median survival after craniotomy of 11 months with an acceptable postoperative mortality of 3%. Survival rates of 1-, 2-, 3-, and 5-year were 46.3%, 24.2%, 14.7%, and 12.5% respectively. Authors concluded that notwithstanding prognosis in metastatic patients remained poor yet, aggressive strategies could improve QoL and occasionally prolong significantly survival.

Full table
Adrenal metastases
Adrenal glands are common sites for metastatic lesions from a variety of neoplasms such as NSCLC (63) with an actual incidence of 10.6% for stage IV patients and occurring up to 59% in autopsy series (26). In a large retrospective series of locally advanced NSCLC, 4.1% presented incidental adrenal masses and 1.6% of them harboured an adrenal metastasis (20). Several retrospective case series about surgical treatment of M1b-adrenal NSCLC patients have been reported (Table 4). Historically, early fascinating results were reported by a multicentre French study (64) involving 43 patients with a median survival of 11 months and with some long-survivor patients. However, 5-year OS was only 7%. On the other hand, the Authors validated feasibility of adrenalectomy in cancer patients without any increase in morbidity or mortality. In a single center experience, Mercier et al. (30) showed a 5-year OS of 23.3% while Pfannschmidt et al. (65) displayed a median OS of 12.9 months. Tanvetyanon et al. (66), in a wide review of literature involving 10 published articles for a total of 114 patients, highlighted a 5-years OS of about 25%. Raz et al. (67), studied 37 patients with isolated adrenal metastases retrospectively (20 of them underwent adrenalectomy), reported a 5-year OS of 34% for patients operatively treated and 0% for patients who underwent medical therapy (P=0.002). Barone et al. (68), in a small series involving 18 patients who underwent adrenalectomy (12 via transabdominal approach and 6 via thoracoabdominal approach), showed a median OS of 31 months with a 3- and 5-year OS of 48.0 and 29.3% respectively. In regard to prognostic factors, Raz et al. (67) firstly reported that ipsilateral metastases of primary lung tumor presented a significant gaining in survival (5-year OS 83% vs. 0%, P=0.003). Moreover, the absence of nodal disease (N0) had a 5-year OS of 52% compared with 0% for patients with N+ disease (P=0.008). On the other hand, no prognostic value according to time-to-relapse was found (synchronous vs metachronous disease: P=0.81). Laterality was considered as a prognostic factor in the evaluation of oligometastatic adrenal disease. In fact, Raz et al. (67) reported 7 patients with ipsilateral adrenal disease and 13 with contralateral one. Patients with ipsilateral presented a 5-year OS 83% compared to 0% for contralateral ones (P=0.003). Synchronous adrenal metastases are thought to be associated with a poor prognosis when compared to metachronous ones. Tanvetyanon et al. (66), pooling 10 publications of NSCLC, revealed that the median OS for patients with synchronous metastases was significantly shorted compared with metachronous ones (12 vs. 31 months, P=0.02), but surprisingly 5-year OS did not differ between two groups (26% vs. 25%). Similar results were published by Mercier et al. (30), who identified disease free interval greater than 6 months as a prognostic factor (Table 5). Concerning with surgical techniques, Barone et al. (68) published a transthoracic access technique the single stage excision of both the primitive neoplasm and of the metastasis. On the other hand, no prospective trials evaluating open versus minimally invasive accesses have been published. Strong et al. (69), in a retrospective study of patients with adrenal metastases, compared 18 patients who underwent open adrenalectomy with 21 who underwent a laparoscopic adrenalectomy. There was no significant difference in OS between the two braces (OA vs. LA), with a 1- and 3-year survival rates of 69% and 41% vs. 58% and 49%, respectively (P=0.96). Minimally invasive approach was associated with a shorter operative time, a lower intraoperative blood loss and a shorter length of hospital stay. However, patients with giant adrenal metastases (greater than 45 mm) presented both inferior survival rates and increased risk of local recurrence if laparoscopically treated (P=0.008, P=0.001). Recently, stereotactic body radiotherapy (SBRT) approaches have provided valid non-invasive alternative options for metastatic adrenal disease with good local control, though less data exists on the efficacy of SBRT and no prospective trials have been published. However, Holy et al. (70) noted a median OS of 23 months in 13 SBRT patients with a 2-year local control rate of 77%. In a small retrospective NSCLC series, Celik et al. (71) reported 1- and 2-year local control rates of 60% and 46.6%, respectively. Differences both in local relapse and OS were found according to time to disease relapse and, in particular, metachronous metastases presented a 2-year OS of 91.2% while synchronous ones 42.8% (P=0.000). Guiou et al. (72), evaluating nine patients with stage IV lung cancer and treated with SBRT, reported an overall RECIST-based response rate of 67% and a 1- and 2-year survival of 52% and 13%, respectively.

Full table

Full table
Other sites
Notwithstanding the propensity for cerebral or adrenal metastases, other sites were described in stage IV NSCLC patients such as bone, liver, axillary nodes or subcutaneous tissue. Collected data come only from small and single-center series (21,31,35,73). Congedo et al. (31) described 53 patients with oligometastatic disease rather than brain and adrenal ones, including bone tissue, liver and contralateral supraclavicular lymph nodes treated with locally aggressive procedures for curative intent. With an acceptable morbidity and 30-day mortality rate, a R0-resection was achieved in 79% with a significant association with overall prognosis (HR =4.75; 95% CI, 1.87–12.10; P=0.001) but the site of organ metastasis did not influence survival. Rarely, oligometastases were described in other organs such as pancreas, spleen, skin, stomach, ear or face tissues as reported in a systematic review by Salah et al. (73). Of 62 enrolled patients, 33 (53%) presented non-visceral solitary metastasis and 29 (47%) a visceral one. The most common sites were bone (n=13), liver (n=9), kidney (n=7) and spleen (n=6). Fifty-eight patients underwent curative resection of primary lung tumor with a median 5-year OS rate of 50%. Patients with a non-visceral metastasis had similar OS compared to patients with a visceral metastasis (5-year survival of 63% vs. 39%, respectively; P=0.30). There was no statistical significance based on a synchronous versus a metachronous presentation (5-year survival 57% vs. 46%, respectively; P=0.79). Moreover, the presence of mediastinal lymphadenopathy (N+ disease) was associated with worse survival if compared with N0 or N1 disease (5-year survival 64% vs. 0%, respectively; P<0.001). An aggressive strategy should be offered also in patients with “uncomfortable” metastases, such as pancreas. DeLuzio et al. (74), in a systematic review involving 32 patients with pancreatic metastases, showed satisfactory outcomes after surgical treatment (OS: 29 months). Studies that have examined the role of surgery for the management of hepatic oligometastases from NSCLC are also limited. There are a few case reports and case series suggesting potential long-term survival after resection of hepatic oligometastases in patients with NSCLC (75) but no conclusions can be drawn from such results.
Conclusions
Oligometastatic lung cancer is characterized by a high clinical heterogeneity associated to innumerable therapeutic strategies. Although an accurate assessment of patients is necessary, published data and reports favour for the adoption of aggressive therapeutic strategies due to the control of primary disease and its distant metastases is a cornerstone and the first prognostic factor to refer to. Oligometastatic disease has become commonplace, especially after the introduction of target therapies with a significant increase of long-survivor patients. Therefore, this implies an adequate knowledge of the expendable resources for the treatment of an oligometastatic patient with curative intent. Some differences should be noted for what concerns brain metastases, where a risk-benefit analysis would be indicated slight propensity towards radiotherapic ablative procedures rather than a surgical approach, especially for the risk long-term disabilities in quality of life. However, locally aggressive strategies present also medical and ethical limits. In fact, in a patient who already underwent a distant metastasectomy, how ethical it is to continue with further demolitions in the name of surgical oncological radicality?
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- International Agency for Research on Cancer EUCAN. Lung cancer factsheets. Available online: http://eco.iarc.fr/eucan/Cancer.aspx?Cancer=18
- Villaruz LC, Kubicek GJ, Socinski MA. Management of non-small cell lung cancer with oligometastasis. Curr Oncol Rep 2012;14:333-41. [Crossref] [PubMed]
- Hanagiri T, Takenaka M, Oka S, et al. Results of a surgical resection for patients with stage IV non-small-cell lung cancer. Clin Lung Cancer 2012;13:220-4. [Crossref] [PubMed]
- Maclean J, Fersht N, Singhera M, et al. Multi-disciplinary management for patients with oligometastases to the brain: results of a 5 year cohort study. Radiat Oncol 2013;8:156. [Crossref] [PubMed]
- Grossi F, Kubota K, Cappuzzo F, et al. Future scenarios for the treatment of advanced non-small cell lung cancer: focus on taxane-containing regimens. Oncologist 2010;15:1102-12. [Crossref] [PubMed]
- American Cancer Society. Cancer Fact and Figures 2017. Available online: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf
- Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995;311:899-909. [Crossref] [PubMed]
- Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev 2010.CD007309. [PubMed]
- Sande TA, Ruenes R, Lund JA, et al. Long-term follow-up of cancer patients receiving radiotherapy for bone metastases: results from a randomised multicentre trial. Radiother Oncol 2009;91:261-6. [Crossref] [PubMed]
- Spiro SG, Rudd RM, Souhami RL, et al. Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax 2004;59:828-36. [Crossref] [PubMed]
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:2895-902. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 2011;12:1004-12. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- David EA, Canter RJ, Chen Y, et al. Surgical Management of Advanced Non-Small Cell Lung Cancer Is Decreasing But Is Associated With Improved Survival. Ann Thorac Surg 2016;102:1101-9. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Kay FU, Kandathil A, Batra K, et al. Revisions to the Tumor, Node, Metastasis staging of lung cancer (8th edition): Rationale, radiologic findings and clinical implications. World J Radiol 2017;9:269-79.
- Shin J, Keam B, Kim M, et al. Prognostic Impact of Newly Proposed M Descriptors in TNM Classification of Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:520-8. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Mordant P, Arame A, De Dominicis F, et al. Which metastasis management allows long-term survival of synchronous solitary M1b non-small cell lung cancer? Eur J Cardiothorac Surg 2012;41:617-22. [Crossref] [PubMed]
- Albain KS, Crowley JJ, LeBlanc M, et al. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol 1991;9:1618-26. [Crossref] [PubMed]
- Griffioen GH, Toguri D, Dahele M, et al. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung Cancer 2013;82:95-102. [Crossref] [PubMed]
- Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol 2010;40:107-11. [Crossref] [PubMed]
- Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2109-21. [Crossref] [PubMed]
- Lussier YA, Khodarev NN, Regan K, et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS One 2012;7:e50141. [Crossref] [PubMed]
- Utley M, Treasure T. Interpreting data from surgical follow-up studies: the role of modeling. J Thorac Oncol 2010;5:S200-2. [Crossref] [PubMed]
- Ashworth A, Rodrigues G, Boldt G, et al. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer 2013;82:197-203. [Crossref] [PubMed]
- Tamura T, Kurishima K, Watanabe H, et al. Characteristics of clinical N0 metastatic non-small cell lung cancer. Lung Cancer 2015;89:71-5. [Crossref] [PubMed]
- Collaud S, Stahel R, Inci I, et al. Survival of patients treated surgically for synchronous single-organ metastatic NSCLC and advanced pathologic TN stage. Lung Cancer 2012;78:234-8. [Crossref] [PubMed]
- Gray PJ, Mak RH, Yeap BY, et al. Aggressive therapy for patients with non-small cell lung carcinoma and synchronous brain-only oligometastatic disease is associated with long-term survival. Lung Cancer 2014;85:239-44. [Crossref] [PubMed]
- Mercier O, Fadel E, de Perrot M, et al. Surgical treatment of solitary adrenal metastasis from non-small cell lung cancer. J Thorac Cardiovasc Surg 2005;130:136-40. [Crossref] [PubMed]
- Congedo MT, Cesario A, Lococo F, et al. Surgery for oligometastatic non-small cell lung cancer: long-term results from a single center experience. J Thorac Cardiovasc Surg 2012;144:444-52. [Crossref] [PubMed]
- Khan AJ, Mehta PS, Zusag TW, et al. Long term disease-free survival resulting from combined modality management of patients presenting with oligometastatic, non-small cell lung carcinoma (NSCLC). Radiother Oncol 2006;81:163-7. [Crossref] [PubMed]
- Daniels M, Wright GM. Complete resection of non-small-cell lung cancer and oligo-metastatic brain disease. ANZ J Surg 2005;75:963-6. [Crossref] [PubMed]
- Yuksel C, Bozkurt M, Yenigun BM, et al. The outcome of bifocal surgical resection in non-small cell lung cancer with synchronous brain metastases: results of a single center retrospective study. Thorac Cardiovasc Surg 2014;62:605-11. [PubMed]
- Endo C, Hasumi T, Matsumura Y, et al. A prospective study of surgical procedures for patients with oligometastatic non-small cell lung cancer. Ann Thorac Surg 2014;98:258-64. [Crossref] [PubMed]
- Iyengar P, Lau S, Donington JS, et al. Local Therapy for Limited Metastatic Non-Small Cell Lung Cancer: What Are the Options and Is There a Benefit? Am Soc Clin Oncol Educ Book 2016;35:e460-7. [Crossref] [PubMed]
- Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin 2003;21:1-23. [Crossref] [PubMed]
- Sheehan JP, Sun MH, Kondziolka D, et al. Radiosurgery for non-small cell lung carcinoma metastatic to the brain: long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg 2002;97:1276-81. [Crossref] [PubMed]
- Grossi F, Scolaro T, Tixi L, et al. The role of systemic chemotherapy in the treatment of brain metastases from small-cell lung cancer. Crit Rev Oncol Hematol 2001;37:61-7. [Crossref] [PubMed]
- Gondi V, Mehta MP. Novel insights into the management of brain metastases. Curr Opin Neurol 2010;23:556-62. [Crossref] [PubMed]
- O'Neill BP, Iturria NJ, Link MJ, et al. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys 2003;55:1169-76. [Crossref] [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-e399S.
- Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 2000;47:291-8. [Crossref] [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [Crossref] [PubMed]
- Mehta MP, Tsao MN, Whelan TJ, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 2005;63:37-46. [Crossref] [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [Crossref] [PubMed]
- Welzel G, Fleckenstein K, Schaefer J, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys 2008;72:1311-8. [Crossref] [PubMed]
- DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology 1989;39:789-96. [Crossref] [PubMed]
- Halasz LM, Uno H, Hughes M, et al. Comparative effectiveness of stereotactic radiosurgery versus whole-brain radiation therapy for patients with brain metastases from breast or non-small cell lung cancer. Cancer 2016;122:2091-100. [Crossref] [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [Crossref] [PubMed]
- Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008;70:510-4. [Crossref] [PubMed]
- Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016;388:2004-14. [Crossref] [PubMed]
- Arrieta O, Villarreal-Garza C, Zamora J, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol 2011;6:166. [Crossref] [PubMed]
- Flannery TW, Suntharalingam M, Regine WF, et al. Long-term survival in patients with synchronous, solitary brain metastasis from non-small-cell lung cancer treated with radiosurgery. Int J Radiat Oncol Biol Phys 2008;72:19-23. [Crossref] [PubMed]
- Hu C, Chang EL, Hassenbusch SJ 3rd, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer 2006;106:1998-2004. [Crossref] [PubMed]
- Iwasaki A, Shirakusa T, Yoshinaga Y, et al. Evaluation of the treatment of non-small cell lung cancer with brain metastasis and the role of risk score as a survival predictor. Eur J Cardiothorac Surg 2004;26:488-93. [Crossref] [PubMed]
- Bella MJ, Kowalewski J, Dancewicz M, et al. Results of surgical treatment of primary lung cancer with synchronous brain metastases. Kardiochir Torakochirurgia Pol 2015;12:14-7. [Crossref] [PubMed]
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1-27. [Crossref] [PubMed]
- Torre M, Barbieri B, Bera E, et al. Surgical therapy in lung cancer with single brain metastasis. Eur J Cardiothorac Surg 1988;2:336-9. [Crossref] [PubMed]
- Bae MK, Yu WS, Byun GE, et al. Prognostic factors for cases with no extracranial metastasis in whom brain metastasis is detected after resection of non-small cell lung cancer. Lung Cancer 2015;88:195-200. [Crossref] [PubMed]
- Bonnette P, Puyo P, Gabriel C, et al. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest 2001;119:1469-75. [Crossref] [PubMed]
- Wroński M, Arbit E, Burt M, et al. Survival after surgical treatment of brain metastases from lung cancer: a follow-up study of 231 patients treated between 1976 and 1991. J Neurosurg 1995;83:605-16. [Crossref] [PubMed]
- Brunt LM, Moley JF. Adrenal incidentaloma. World J Surg 2001;25:905-13. [Crossref] [PubMed]
- Porte H, Siat J, Guibert B, et al. Resection of adrenal metastases from non-small cell lung cancer: a multicenter study. Ann Thorac Surg 2001;71:981-5. [Crossref] [PubMed]
- Pfannschmidt J, Schlolaut B, Muley T, et al. Adrenalectomy for solitary adrenal metastases from non-small cell lung cancer. Lung Cancer 2005;49:203-7. [Crossref] [PubMed]
- Tanvetyanon T, Robinson LA, Schell MJ, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol 2008;26:1142-7. [Crossref] [PubMed]
- Raz DJ, Lanuti M, Gaissert HC, et al. Outcomes of patients with isolated adrenal metastasis from non-small cell lung carcinoma. Ann Thorac Surg 2011;92:1788-92. [Crossref] [PubMed]
- Barone M, Di Nuzzo D, Cipollone G, et al. Oligometastatic non-small celllungcancer (NSCLC): adrenalmetastases. Experience in a single institution. Updates Surg 2015;67:383-7. [Crossref] [PubMed]
- Strong VE, D'Angelica M, Tang L, et al. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol 2007;14:3392-400. [Crossref] [PubMed]
- Holy R, Piroth M, Pinkawa M, et al. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol 2011;187:245-51. [Crossref] [PubMed]
- Celik E, Semrau R, Baues C, et al. Robot-assisted Extracranial Stereotactic Radiotherapy of Adrenal Metastases in Oligometastatic Non-small Cell Lung Cancer. Anticancer Res 2017;37:5285-91. [PubMed]
- Guiou M, Mayr NA, Kim EY, et al. Stereotactic body radiotherapy for adrenal metastases from lung cancer. J Radiat Oncol 2012;1:155-63. [Crossref]
- Salah S, Tanvetyanon T, Abbasi S. Metastatectomy for extra-cranial extra-adrenal non-small cell lung cancer solitary metastases: systematic review and analysis of reported cases. Lung Cancer 2012;75:9-14. [Crossref] [PubMed]
- DeLuzio MR, Moores C, Dhamija A, et al. Resection of oligometastatic lung cancer to the pancreas may yield a survival benefit in select patients--a systematic review. Pancreatology 2015;15:456-62. [Crossref] [PubMed]
- Ileana E, Greillier L, Moutardier V, et al. Surgical resection of liver non-small cell lung cancer metastasis: a dual weapon? Lung Cancer 2010;70:221-2. [Crossref] [PubMed]


