Surveillance versus esophagectomy in esophageal cancer patients with a clinical complete response after induction chemoradiation
Introduction
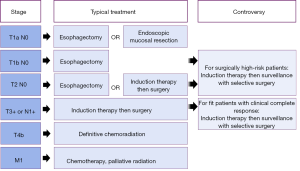
The treatment of thoracic esophageal or gastroesophageal junction cancer varies by stage. The earliest stage cancers are treated with surgery (1): either endoscopic resection if the tumor is confined to the mucosa, or esophagectomy if there is evidence of deeper invasion. Patients with locally advanced cancers or evidence of regional lymph node spread have been shown in randomized controlled trials to benefit from induction chemoradiation followed by an esophagectomy as compared to esophagectomy alone (2). Patients with inoperable or unresectable cancers are palliated with definitive chemotherapy or chemoradiation. This typical treatment paradigm, outlined in Figure 1, is well-established for adenocarcinoma patients who are good operative candidates and is supported by esophageal cancer management guidelines from multiple major oncologic groups (3,4). Details may differ for some squamous cell cancer patients, particularly those with cervical esophageal cancer for whom chemoradiation alone is often preferred. New areas of controversy have emerged as chemotherapy and radiation treatments have improved and our field focuses more on individualized medicine. The standard pathway is commonly debated for those Siewert I and II adenocarcinoma patients who have an apparent complete response to induction chemoradiation.
High-level evidence supports restaging following neoadjuvant therapy to rule out interval development of distant metastases prior to committing to the more risky and morbid surgical resection. As a result, preferred re-staging strategies often include clinical re-evaluation, CT or PET/CT scanning, and possibly endoscopy to assess the response of the primary tumor to treatment. The Society of Thoracic Surgeons (STS) and National Comprehensive Cancer Network (NCCN) recommendations are to proceed with esophagectomy as long as: there is no evidence of distant metastatic spread, the cancer remains locoregionally resectable, and the patient remains a favorable operative candidate (3,4). For individuals with evidence of persistent localized disease on post-induction restaging who will tolerate an esophagectomy, these recommendations are not controversial: the standard treatment pathway is likely to improve their long-term survival (5). In recent years, however, the role of universal surgery following chemoradiation for locally advanced esophageal cancer has been called into question, especially for patients who are loosely defined as “marginal” operative candidates at diagnosis or those in whom residual cancer cannot be demonstrated on restaging. For patients who meet both conditions: high risk and apparent clinical complete response, the appeal of a “watch and wait” strategy seems obvious.
This consideration for active surveillance after induction therapy has been influenced by the treatment protocols for cervical esophageal squamous cancers, for which the tide has shifted and the standard of care has become definitive chemoradiation, followed by resection if there is evidence of tumor persistence or recurrence (6-8). European trials in predominantly squamous cell cancers have demonstrated that a similar strategy could potentially be successfully employed for lower thoracic esophageal squamous cell cancers (9,10). Consequently, an interest in esophageal-preserving therapy has emerged and delayed operations, or surveillance strategies with selective salvage esophagectomies, have become more common (11) due to both patient and provider preferences.
In clinical management, this has created an area of ambiguity for patients with thoracic esophageal cancers who have an apparent complete response after induction therapy, as well as those with earlier stage disease considered high-risk operative candidates due to factors such as age or comorbid status. These areas of controversy are highlighted in Figure 1, organized by stage and contrasted with usual care. In this article, we aim to review the existing evidence that may contribute to nuanced decision-making for these less straight-forward patients, focusing on the central question: what is optimal care if a patient appears to have a complete response on restaging?
Identifying this patient population
To set the stage for this discussion, it is first important to define the terms clinical complete response (cCR) and pathologic complete response (pCR), as well as to distinguish between the two. A cCR is the absence of demonstrable persistent cancer with the non-operative diagnostic tools available following induction chemoradiation. In the literature, there is some variability in this definition based on the modalities employed for restaging a patient. These include: repeat endoscopic biopsies that are negative for residual tumor, no apparent cancer in the esophageal wall or lymph nodes on endoscopic ultrasound (EUS) or CT, a percentage reduction in PET SUV above various empiric thresholds (most commonly 30–50%), a resolution of PET SUV uptake back to physiologic levels or in a pattern consistent with post-treatment esophagitis, or a composite clinical response in which there is no apparent residual cancer on multiple modalities (12-14).
A pCR is the absence of viable tumor cells in the esophagectomy specimen and all associated nodes as determined by a pathologist following surgical resection. Depending on the series, a pCR can be seen in 11–56%, though this favorable outcome is generally reported in the range of 20–30% of patients, and has consistently been associated with improved overall survival (15-17). A cCR is suggestive of a pCR, but there is not perfect correlation as no current staging modality can definitively exclude the presence of residual microscopic disease.
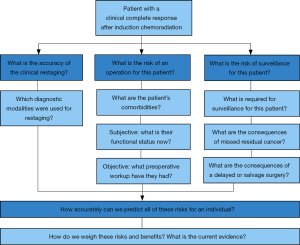
As there have been improvements in care of esophageal cancer patients and increasing use of neoadjuvant chemoradiation in locally advanced disease, thoracic oncologists of all specialties are seeing patients with cCRs more frequently. A provider’s choice regarding whether or not to recommend esophagectomy in this patient population can be based on three primary questions:
- What is the accuracy of the clinical restaging?
- What is the risk of an operation for this patient?
- What is the risk of surveillance for this patient?
An outline of this clinical approach and related considerations is depicted in Figure 2, and provides the structure of the remainder of this review.
The accuracy of clinical re-staging
Our ability to accurately predict whether a patient has been effectively cured with induction chemoradiation alone is a center point in this discussion. Specifically, we would like to quantify the likelihood of a pCR in a patient with a cCR.
The recommended diagnostic modalities frequently used for restaging include endoscopy with surface biopsies, EUS with directed FNA to deeper areas of concern in the esophagus and adjacent nodes, CT alone, and PET/CT. CT scanning of the chest and abdomen is an option, though the ability to distinguish between residual tumor and post-treatment inflammation in this setting is less accurate with CT alone than with the addition of PET (18). The utility of repeat EUS at this phase is debatable since the sensitivity and specificity for identifying residual cancer has been demonstrated to be low. Finally, there has been some interest in the use of MRI to identify residual tumor, although this data is very preliminary. Here, we will discuss the reliability of five modalities: endoscopy, EUS, PET, CT, and MRI, all considered independently and in various combinations, for predicting a pCR following neoadjuvant chemotherapy. A summary of selected studies can be found in Table 1.

Full table
Endoscopy and EUS
Even prior to neoadjuvant treatment, endoscopy and EUS have some limitations in their ability to accurately stage tumors, most notably in detecting microscopic nodal metastases. Several studies have shown non-trivial rates of unanticipated nodal disease in initial staging of both early and locally advanced cancers that are treated surgically: 24% in cT1, 39–55% in cT2, and 78% in cT3 (19,20). Post-treatment effects, such as ongoing inflammation in the esophageal wall, enlarged reactive lymph nodes, and radiation-induced alterations in the echogenicity of lymph nodes seem to further limit reliable restaging with these techniques alone.
Single center studies have unsurprisingly shown poor correlation between restaging with endoscopic modalities (including endoscopic assessment, endoscopy with biopsies, and EUS) and actual pathologic stage after resection with negative predictive values (NPV) and accuracy assessments around 70% (17,21,22). Many additional studies examine the ability of these modalities to predict tumor and nodal responses separately, without directly assessing a combined pCR. A recently performed meta-analysis (15) of these studies found pooled estimates for tumor staging of sensitivity and specificity of endoscopic biopsy to be 34.5% (26.0–44.1%) and 91% (85.6–94.5%), and of EUS to be 96.4% (91.7–98.5%) and 10.9% (3.5–29.0%), respectively. When examining the pooled estimates for nodal disease, the sensitivity and specificity of EUS were similarly unimpressive at 62% (46.0–75.7%) and 56.7% (41.8–70.5%). They also specifically examined the NPV of these tests. Endoscopic biopsy for tumor staging had an overall NPV of 47% and EUS for nodal staging had an overall NPV of 77%, and were noted to be better for squamous cell cancers (72% and 92%) than adenocarcinomas (35% and 65%) on subgroup analysis. A portion of patients will have a complete primary tumor response with residual nodal disease, though, and for the population for which we are considering active surveillance rather than resection, it is really the composite outcome that matters.
Endoscopic techniques, especially with biopsies, can provide very useful information if evidence of post-induction residual cancer is detected: those patients, as a group, have no non-surgical curative therapy available and they will have improved survival with an esophagectomy. In contrast, an inability to demonstrate residual cancer with endoscopic modalities alone does not yet offer justification to avoid an operation for a typical-risk patient.
PET and PET/CT
PET as a restaging modality has an advantage in that it reflects changes in tissue metabolism that may precede the observable structural changes that might be noted on CT or EUS when assessing response (14,18). A couple of systematic reviews and meta-analyses have been performed examining the question of PET accuracy in predicting a pathologic response. It is worth noting that these meta-analyses were done with the explicit goal of assessing whether a patient is responding vigorously or poorly to induction therapy and whether that patient should stop induction early and proceed to surgery if the response was poor. This is a far different aim from deciding whether to avoid or delay a potentially unnecessary operation in patients with a complete response. Consequently, these meta-analyses included studies of PET performed at any time point after initiation of chemoradiation—sometimes during the ongoing therapy. The studies also differed in the way they defined a histopathologic response, but most commonly they used <10% viable residual tumor cells, with few studies examining the ability to predict a pCR. Even in this setting, the pooled sensitivities and specificities of PET were only 67–70% and 68–70%, respectively (13,14). Although these publications include the largest numbers of patients, these results should be interpreted with caution in our discussion, because the presence of residual microscopic disease would suggest surgery rather than surveillance.
To get a better sense of PET accuracy in our specific population of interest, we turn instead to individual studies that explicitly focused on PET to evaluate for pCR. Two studies have prospectively compared PET or PET/CT to other restaging modalities in small cohorts of patients, of which 35–45% of patients had a pCR after esophagectomy. They found that PET or PET/CT predicted a complete response accurately in 71–88% of these patients and had a NPV of 64–94%, which is much higher than the accuracy and NPV of CT (58–73%, 44–71%) or EUS (70–71%, 67%) respectively (17,22). A subsequent small retrospective study (16) examining PET or PET/CT did not reveal the same encouraging predictiveness, with an accuracy of 56% and NPV of only 35%. This heterogeneity reflects the variability seen in the meta-analyses mentioned above, and may be due to the small sample size of the studies, the use of PET with or without CT information, or the variability in definition of what constitutes a positive or negative restaging scan.
MRI
Use of MRI in restaging of esophageal cancer is not standard, but the modality has been shown to be useful in some other gastrointestinal cancers, such as rectal cancer. Because our current tests are imperfect and there is interest in individualizing treatment based on clinical response, investigators have conducted a small pilot study of diffusion weighted MRI for predicting pCR. Scans from 20 patients undergoing induction chemoradiation followed by surgery, of which 4/20 (20%) had a pCR, were examined and the investigators were able to identify an imaging threshold at which there was high sensitivity and specificity of MRI in this small subset of patients (23). Obviously larger studies are needed to confirm the findings, and the combined value of MRI and other restaging modalities has not been explored.
Composite clinical responses
Perhaps the most useful studies are those that examine the combined ability of multiple restaging modalities to predict a pCR. This approach is more pragmatic and provides the closest approximation of a provider’s actual clinical approach to decision making. Several studies have examined various combinations of PET, CT, endoscopy, and esophagography in cohorts of 60–280 patients and have found a physician-assessed clinical response to have an accuracy between 46–79% and NPV between 31–74% (17,24,25). Again, this variability may reflect the combination of modalities used, the prevalence of pCRs in their source populations, or different definitions of what constitutes a positive vs. negative screening result.
Risk prediction model
The group at MD Anderson performed a retrospective study of 322 patients, of which 70 had a pCR, and developed a nomogram that incorporates the patient characteristics of sex, tumor grade, and baseline tumor staging with post-treatment data from PET scanning and endoscopic biopsy results to predict the likelihood of a pCR. The corrected AUC for this model was 0.70 (26), which is considered ‘fair’ accuracy. While this model has not been validated in another patient cohort, the group has shown that dichotomized nomogram scores also correlate with overall and disease-free survival outcomes (27).
There certainly are consequences to choosing incorrectly whether to operate on or observe a specific patient. There are essentially competing risks of the two strategies: the risks of surgery, including operative morbidity and mortality, and the opposing risks of surveillance, including missed residual disease that may progress to unresectability or metastasize. The threshold needed to favor a surveillance strategy depends on the balance of competing surgical risks for a patient. Risk prediction models and probabilities based on composite clinical metrics can inform the provider of the likelihood of a pCR, and provide a background upon which to consider the risks of an esophagectomy or surveillance strategy.
Predicting the risk of an operation
A futile operation is one that results in perioperative death or debilitating morbidity, or alternatively does not prolong survival over what would be gained without surgery. When a patient is diagnosed with locally advanced esophageal cancer, they frequently undergo their staging workup and a multidisciplinary clinical evaluation to determine if they are a candidate for trimodality therapy or if they should get definitive chemoradiation instead. This workup often includes subjective and objective measures of health status and is meant to risk-stratify patients for surgery, ideally identifying those for whom an esophagectomy may be a prohibitively high-risk venture. This workup includes a history and physical, functional status assessment, as well as any additional indicated cardiovascular and pulmonary function testing. Despite preoperative evaluation, esophagectomy is an operation that carries a morbidity rate between 15–40% and a mortality rate usually less than 5% but occasionally up to 10% (28). In a recent large series using the STS database, the morbidity is 33% and the mortality is 3% (29).
Several national databases and risk calculators have been created in an attempt to predict risk of an operation by entering baseline patient factors, and subsequently tracking outcomes based on tumor, patient, and hospital characteristics. These databases are voluntary and the data entered is audited for accuracy to a varying degree. One study that compared the STS database and the National Surgery Quality Improvement Program (NSQIP) database found that rates of complications differed significantly within a single institution based on whether a comprehensive (STS) or partial sampling (NSQIP) database was used for assessment. For example, the observed rates of pneumonia and mortality in esophagectomy varied by 3-fold depending on whether all cases were included or incomplete subsets were utilized (30). Another study compared the STS database to both the NSQIP and National Inpatient Sample (NIS) databases and found that the national mortality rates of esophagectomy varied by greater than 2-fold, depending on the database used. There were also significant and meaningful differences between the databases in hospital length of stay (28). This variability in captured morbidity and mortality obviously has implications for reliably predicting outcomes using risk calculators that are numerically based on these databases. To date, there is no widely agreed upon method to predict the risk of mortality or major morbidity for esophagectomy.
Predicting the risk of surveillance
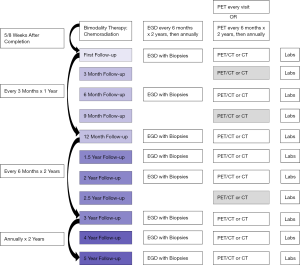
To advocate for observation after chemoradiation in selected patients, an effective surveillance strategy needs to be in place, and the risks of a delayed operation need to be reasonable. The goal is to reliably detect a local recurrence prior to the cancer metastasizing or prior to it becoming locally unresectable by invading adjacent structures. Surveillance strategies differ nationally, with the primary variations found in the frequency of use for PET and endoscopy. To provide a basis for this discussion, an outline of a possible surveillance strategy is provided in Figure 3 that combines recommendations from the existing literature (3,31).
Early risks
A small number of studies have examined the risks of a short delay prior to esophagectomy. One study (32) examined 325 patients who participated in the CROSS trial, and demonstrated that a delay of up to twelve weeks was associated with a very small increased risk of complications (OR 1.2 for each week delay after 6.5 weeks), though these patients also had an increased likelihood of a pCR (OR 1.35 per week). This led the authors to conclude that the observed findings allow for safe testing of a ‘wait-and-see strategy’. Another single institution study (33) reviewed 266 patients who underwent esophagectomy sooner than or later than 8 weeks after completion of neoadjuvant treatment. They found there was no significant difference in morbidity and mortality rates, the difficulty of the operation based on surrogate measures, or the rate of pCR. They did note that there was a slight nonsignificant trend towards more anastomotic leaks (16% vs. 11%), more pulmonary complications (35% vs. 31%), higher mortality (3% vs. 2%), and shorter overall survival (39 vs. 53 months) for the delayed vs. early group, though this study explicitly excluded patients choosing initial surveillance and needing an eventual salvage esophagectomy, so membership in the delayed group may have signalled higher medical risk at baseline.
Late risks
Patients who undergo definitive chemoradiation or have a cCR after neoadjuvant treatment and choose not to have immediate surgery may develop a local or regional recurrence and thus become a candidate for a salvage esophagectomy. Older studies of salvage procedures showed high morbidity and mortality rates with anastomotic leaks occurring in 21–38% of patients and 30-day mortality ranging widely between 4–33%, but with 5-year survival rates that were similar to that of patients undergoing planned surgeries (34). Subsequent studies, however, have actually started to show more comparable rates of major postoperative events, perioperative mortality, and median survival. One retrospective study of 65 patients undergoing a salvage operation compared to 521 patients undergoing planned trimodality therapy found leak rates were 18.5% vs. 11.3%, 30-day mortality was 3.1% vs. 2.9%, 3-year survival was 48% vs. 57%, and 5-year survival was 32% vs. 45% for the two groups respectively (35). Another small matched study actually showed a trend towards better survival in patients who declined initial surgery after neoadjuvant chemoradiation, and got a salvage esophagectomy if they recurred locally, versus the strategy of getting a planned operation: the median survival was 58 vs. 51 months (36). This shift may reflect improvements in operative technique and perioperative care, lower doses of neoadjuvant radiation usage, or better patient selection with multidisciplinary care (34). Regardless, the improved outcomes in patients undergoing salvage procedures after surveillance provides encouragement that this may be a viable strategy for a subset of patients.
Risk of locoregional vs. systemic recurrence
Understanding the risk of a patient developing a locoregional recurrence versus systemic disease is useful for considering two very different circumstances in which an operation may be futile: first, in the setting of a pCR where a patient may be at greater risk of death from competing comorbidities or the morbidity of the operation; and second, where the patient has undetected distant micrometastatic disease where local control with an esophagectomy will not improve survival. To quantify this risk, we examine patterns of recurrence after chemoradiation treatment.
One source of this information is the natural history of the cohort of patients undergoing planned definitive chemoradiation. A single institution study of 276 patients who underwent definitive chemoradiation found that 70% of patients had a cCR at the time of their first follow up. Of these complete clinical responders, 47% never developed a relapse. Among the 53% of the initial cohort that did recur: 43% had a local recurrence only, meaning they could be potential candidates for salvage esophagectomy, while 40% had distant metastases only and an operation would have been futile, and 17% had both a local recurrence and distant metastases detected simultaneously. In this last subpopulation, it is unclear whether upfront surgery would have prevented metastatic spread. Of the patients who were candidates for salvage esophagectomy, their corresponding survival was quite good: median, 58.6 months; 3-year, 61%; and 5-year, 45%. An impressive 98% of the relapses developed within 3 years, indicating this is the period where regular surveillance is most important if the patient is considered a candidate for additional treatment (31).
Another population to consider is the cohort of patients who refuse an operation when they have a cCR. One study evaluated a group of 61 such patients who declined an esophagectomy but were trimodality-eligible at their institution and found that 54% had recurrences, and the distribution was very similar to the cohort undergoing planned definitive chemoradiotherapy: approximately 40% were local (nearly all patients were able to undergo salvage esophagectomy) and 60% were metastatic. The overall 5-year survival rate was 58% (12).
Existing evidence
There is currently no randomized controlled trial that directly compares surveillance to surgery in patients who have had a cCR to neoadjuvant treatment. Perhaps the most well-known data comes from the RTOG 0246 trial, which was a non-randomized phase II study that aimed to study selective esophagectomy in patients who had residual disease after induction chemoradiation or who developed progressive or recurrent disease while undergoing surveillance. This study enrolled 41 eligible patients: all of whom underwent standard pretreatment staging (endoscopy, EUS, CT of the chest and abdomen with or without PET), were deemed to have resectable disease, and received two cycles of induction chemotherapy followed by induction chemoradiation. If the patients had evidence or suspicion of residual disease on restaging, they were considered for esophagectomy, otherwise they underwent a multimodality surveillance strategy. After neoadjuvant treatment, 18/41 underwent selective esophagectomy for persistent cancer and 23/41 did not have proof of persistence and were thus observed. During surveillance, an additional 3 patients were found to have suspicion of recurrent disease and ultimately had an operation. The 1-year survival rate for this entire enrolled cohort was 71%, and they did not observe an increased operative morbidity or mortality. The authors had suggested before the trial that a 1-year survival threshold of 77% or higher would be incentive to move this strategy forward to a randomized trial. The threshold survival rate was not reached, but the early data was encouraging for the use of a selective surveillance strategy (37). A follow up report of these patients indicated that a cCR occurred in 15 patients with 5-year survival of 53% and 7-year survival of 47%. Esophagectomy was avoided in half of the patients in the trial, demonstrating a potential to selectively operate in these patients with good short and long-term outcomes (38).
Several additional retrospective reviews have examined the question of surveillance versus surgery, but these have significant limitations with regards to patient selection. One group performed an intention-to-treat case-control study looking retrospectively at a total of 222 patients with a cCR. In this study, 59 patients underwent initial post-induction surveillance and were matched 1:2 with controls undergoing an immediate operation. They found evidence of residual cancer in 34.6% of pathologic specimens, and noted shorter survival (31 vs. 83 months) and faster locoregional recurrence for the surveillance group. It is worth noting that while these groups were matched on important patient and tumor characteristics, they were not matched on specific comorbidities that may have influenced operative decisions. Patients were preferentially assigned to surveillance if they were treated at low volume centers, whereas surgery was proposed and patients made a decision regarding their treatment plan after a risk-benefit discussion at high volume, tertiary referral centers (39). Given the data supporting improved outcomes for patients treated at high-volume cancer centers in general (40), it is difficult to interpret how much of the observed difference in survival was due to the effects of upfront surgery and how much is attributable to other factors.
Perhaps the largest published retrospective study is a National Cancer Database study that used propensity score matching to compare 1,774 matched pairs of patients who underwent induction chemoradiation for stage II/III esophageal cancer with or without subsequent esophagectomy. In the overall cohort, esophagectomy substantially improved survival (32.5 vs. 14.2 months), but this study did not specifically examine patients based on clinical response. Also, importantly, it is likely that the authors would have excluded many of the older, frailer patients by way of creation of their propensity score: patients for whom a more nuanced discussion of risks and benefits is merited. This study is also at risk of substantial confounding by indication (41).
One interesting study did specifically focus on the older individuals that are so often excluded from trials—the authors examined esophagectomy versus surveillance in patients over 70 in a study of 56 patients after chemoradiation, of which 25 had a cCR. Six of those patients underwent an operation and four truly had a pCR. Survival was similar for those undergoing an operation vs. surveillance: the whole cohort with cCRs had a median survival of 47 months (61 months for those undergoing an upfront surgery and 29 months for those who had a salvage operation) versus 46 months for the subset that did not undergo resection. Despite this being a very small retrospective study subject to treatment selection bias, there is a suggestion that overall there may not be a survival advantage to a non-selective progression to surgery in this particular population (42).
Quality of life considerations
Numerous studies have looked at health-related quality of life (HRQOL) after esophagectomy, using metrics that explore individual symptoms, a variety of functional scales, or global assessments. Essentially all have shown that patients experience a decrease in multiple aspects of quality of life in the short-term following an esophagectomy, though HRQOL may recover to baseline values over time. The decreases can be more substantial in patients who experience major complications and, in fact, poor HRQOL after the immediate post-operative dip is associated with worse overall survival. The most significant areas where surgical patients suffer are predictably related to loss of their stomach’s normal function: eating problems, reflux symptoms, loss of appetite, and diarrhea. However, these patients also had worse scores with general health measures like fatigue and dyspnea, and experienced lower overall physical function, vitality scores, and health perception than controls long-term (43). Notably, organ preservation in esophageal cancer patients has been shown to be predictive of global measures of HRQOL (44).
Summary
In the context of all of this data, the question remains for the thoracic surgeon: what is the best course of treatment for the individual patient who presents to clinic with an apparent cCR after induction therapy for a distal esophageal adenocarcinoma?
The answer to this question of surveillance versus surgery is unfortunately unlikely to be found by way of a prospective randomized controlled trial. Patients and providers often have preferences regarding their personal treatment strategy based on perceived risks or quality of life considerations that may limit enrollment of a sufficient number of patients to a classic randomized trial. Additionally, these trials are often not pragmatic, and inclusion and exclusion criteria may not appropriately represent the range of patients seen in the typical thoracic practice. Therefore, even if an adequate number of patients could be accrued for randomization, a formal trial may still leave providers uncertain as to how to apply the results to an individual based on the patient’s health or age, the center’s surgical outcomes, the imaging modalities used for staging, or the pretreatment tumor characteristics.
Furthermore, there is likely not a single best answer for the entire group of patients. In critically evaluating all of this available evidence, it is apparent that our ability to predict the likelihood of true pCR, the risk of an operation, and the risks associated with a surveillance strategy are less than ideal. Consequently, we try to weigh the balance of estimated risks for an individual—both from the surgeon and patient perspectives. For a fit patient who is less likely than others to have major operative complications, a risk of persistent cancer in the range of 30–40% may be too high, even with an aggressive surveillance strategy and plan for salvage operation if recurrence is detected. Avoiding surgery and surgical complications may not be worth the risk of progression to unresectability or metastasis, or the burden of anxiety about cancer recurrence that can occur with organ preservation strategies. Conversely, in the older or sicker patient who has more competing morbidities, a 60–70% chance that the cCR patient truly has a pCR may be good enough: when one considers the fact that if there is residual cancer, it is likely to become metastatic half the time and therefore not be treatable by an esophagectomy, which would come at increased risk of complications with numerous quality of life sacrifices. For patients that fall in the middle of this spectrum, the clinical decision is more complicated. No studies currently capture all of these patient-important factors, so clinical judgment and experience remain at the center of these decisions which results in wide variability of care. Accumulation of additional rich observational patient data from multiple sites with the goal of improving risk prediction or enhancing the foundation for a shared decision-making process may be the best alternative strategy for refining the treatment pathways for these patients, especially if subgroups can be identified for whom one treatment strategy or the other is likely to be preferable.
Acknowledgements
Funding: TR Semenkovich was supported by National Institutes of Health (NIH) Grant Number 2T32HL7776-21, a Barnes Jewish Hospital Foundation Grant, and the Division of Cardiothoracic Surgery at Washington University in St. Louis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- National Comprehensive Cancer Network I. NCCN Guidelines Version 1.2017. 2017.
- Little AG, Lerut AE, Harpole DH, et al. The Society of Thoracic Surgeons practice guidelines on the role of multimodality treatment for cancer of the esophagus and gastroesophageal junction. Ann Thorac Surg 2014;98:1880-5. [Crossref] [PubMed]
- Vincent J, Mariette C, Pezet D, et al. Early surgery for failure after chemoradiation in operable thoracic oesophageal cancer. Analysis of the non-randomised patients in FFCD 9102 phase III trial: Chemoradiation followed by surgery versus chemoradiation alone. Eur J Cancer 2015;51:1683-93. [Crossref] [PubMed]
- Takes RP, Strojan P, Silver CE, et al. Current trends in initial management of hypopharyngeal cancer: the declining use of open surgery. Head Neck 2012;34:270-81. [Crossref] [PubMed]
- Zenda S, Kojima T, Kato K, et al. Multicenter Phase 2 Study of Cisplatin and 5-Fluorouracil With Concurrent Radiation Therapy as an Organ Preservation Approach in Patients With Squamous Cell Carcinoma of the Cervical Esophagus. Int J Radiat Oncol Biol Phys 2016;96:976-84. [Crossref] [PubMed]
- Cao CN, Luo JW, Gao L, et al. Primary Radiotherapy Compared With Primary Surgery in Cervical Esophageal Cancer. JAMA Otolaryngol Head Neck Surg 2014;140:918. [Crossref] [PubMed]
- Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [Crossref] [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation With and Without Surgery in Patients With Locally Advanced Squamous Cell Carcinoma of the Esophagus. J Clin Oncol 2005;23:2310-7. [Crossref] [PubMed]
- Hofstetter W. Current and future options for treating esophageal cancer: a paradigm shift toward organ-sparing therapies. Tex Heart Inst J 2012;39:846-7. [PubMed]
- Taketa T, Correa AM, Suzuki A, et al. Outcome of Trimodality-Eligible Esophagogastric Cancer Patients Who Declined Surgery after Preoperative Chemoradiation. Oncology 2012;83:300-4. [Crossref] [PubMed]
- Kwee RM. Prediction of Tumor Response to Neoadjuvant Therapy in Patients with Esophageal Cancer with Use of 18 F FDG PET: A Systematic Review. Radiology 2010;254:707-17. [Crossref] [PubMed]
- Chen YM, Pan XF, Tong LJ, et al. Can 18F-fluorodeoxyglucose positron emission tomography predict responses to neoadjuvant therapy in oesophageal cancer patients? A meta-analysis. Nucl Med Commun 2011;32:1005-10. [Crossref] [PubMed]
- van Rossum PS, Goense L, Meziani J, et al. Endoscopic biopsy and EUS for the detection of pathologic complete response after neoadjuvant chemoradiotherapy in esophageal cancer: a systematic review and meta-analysis. Gastrointest Endosc 2016;83:866-79. [Crossref] [PubMed]
- McLoughlin JM, Melis M, Siegel EM, et al. Are patients with esophageal cancer who become PET negative after neoadjuvant chemoradiation free of cancer? J Am Coll Surg 2008;206:879-86; discussion 886-7. [Crossref] [PubMed]
- Kim MK, Ryu JS, Kim SB, et al. Value of complete metabolic response by 18F-fluorodeoxyglucose-positron emission tomography in oesophageal cancer for prediction of pathologic response and survival after preoperative chemoradiotherapy. Eur J Cancer 2007;43:1385-91. [Crossref] [PubMed]
- Westerterp M, van Westreenen HL, Reitsma JB, et al. Esophageal Cancer: CT, Endoscopic US, and FDG PET for Assessment of Response to Neoadjuvant Therapy—Systematic Review. Radiology 2005;236:841-51. [Crossref] [PubMed]
- Crabtree TD, Yacoub WN, Puri V, et al. Endoscopic Ultrasound for Early Stage Esophageal Adenocarcinoma: Implications for Staging and Survival. Ann Thorac Surg 2011;91:1509-15; discussion 1515-6. [Crossref] [PubMed]
- Stiles BM, Mirza F, Coppolino A, et al. Clinical T2-T3N0M0 esophageal cancer: the risk of node positive disease. Ann Thorac Surg 2011;92:491-6; discussion 496-8. [Crossref] [PubMed]
- Schneider PM, Metzger R, Schaefer H, et al. Response Evaluation by Endoscopy, Rebiopsy, and Endoscopic Ultrasound Does Not Accurately Predict Histopathologic Regression After Neoadjuvant Chemoradiation for Esophageal Cancer. Ann Surg 2008;248:902-8. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Ohja B, et al. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg 2005;129:1232-41. [Crossref] [PubMed]
- van Rossum PS, van Lier AL, van Vulpen M, et al. MRI in esophageal cancer Diffusion-weighted magnetic resonance imaging for the prediction of pathologic response to neoadjuvant chemoradiotherapy in esophageal cancer. Radiother Oncol 2015;115:163-70. [Crossref] [PubMed]
- Cheedella NKS, Suzuki A, Xiao L, et al. Association between clinical complete response and pathological complete response after preoperative chemoradiation in patients with gastroesophageal cancer: analysis in a large cohort. Ann Oncol 2013;24:1262-6. [Crossref] [PubMed]
- Liu S-L, Xi M, Yang H, et al. Is There a Correlation Between Clinical Complete Response and Pathological Complete Response After Neoadjuvant Chemoradiotherapy for Esophageal Squamous Cell Cancer? Ann Surg Oncol 2016;23:273-81. [Crossref] [PubMed]
- Ajani JA, Correa AM, Hofstetter WL, et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol 2012;23:2638-42. [Crossref] [PubMed]
- Lin SH, Wang J, Allen PK, et al. A nomogram that predicts pathologic complete response to neoadjuvant chemoradiation also predicts survival outcomes after definitive chemoradiation for esophageal cancer. J Gastrointest Oncol 2015;6:45-52. [PubMed]
- Lapar DJ, Stukenborg GJ, Lau CL, et al. Differences in reported esophageal cancer resection outcomes between national clinical and administrative databases. J Thorac Cardiovasc Surg 2012;144:1152-7. [Crossref] [PubMed]
- Society of Thoracic Surgeons General Thoracic Surgery Database Task Force. The Society of Thoracic Surgeons Composite Score for Evaluating Esophagectomy for Esophageal Cancer. Ann Thorac Surg 2017;103:1661-7. [Crossref] [PubMed]
- Allen MS, Blackmon S, Nichols FC, et al. Comparison of Two National Databases for General Thoracic Surgery. Ann Thorac Surg 2015;100:1155-61; discussion 1161-2. [Crossref] [PubMed]
- Sudo K, Xiao L, Wadhwa R, et al. Importance of surveillance and success of salvage strategies after definitive chemoradiation in patients with esophageal cancer. J Clin Oncol. 2014;32:3400-5. [Crossref] [PubMed]
- Shapiro J, van Hagen P, Lingsma HF, et al. Prolonged Time to Surgery After Neoadjuvant Chemoradiotherapy Increases Histopathological Response Without Affecting Survival in Patients With Esophageal or Junctional Cancer. Ann Surg 2014;260:807-13; discussion 813-4. [Crossref] [PubMed]
- Kim JY, Correa AM, Vaporciyan AA, et al. Does the Timing of Esophagectomy After Chemoradiation Affect Outcome? Ann Thorac Surg 2012;93:207-12; discussion 212-3. [Crossref] [PubMed]
- Swisher SG, Marks J, Rice D. Salvage esophagectomy for persistent or recurrent disease after definitive chemoradiation. Ann Cardiothorac Surg 2017;6:144-51. [Crossref] [PubMed]
- Marks JL, Hofstetter W, Correa AM, et al. Salvage Esophagectomy After Failed Definitive Chemoradiation for Esophageal Adenocarcinoma. Ann Thorac Surg 2012;94:1126-32; discussion 1132-3. [Crossref] [PubMed]
- Taketa T, Xiao L, Sudo K, et al. Propensity-Based Matching between Esophagogastric Cancer Patients Who Had Surgery and Who Declined Surgery after Preoperative Chemoradiation. Oncology 2013;85:95-9. [Crossref] [PubMed]
- Swisher SG, Winter KA, Komaki RU, et al. A Phase II study of a paclitaxel-based chemoradiation regimen with selective surgical salvage for resectable locoregionally advanced esophageal cancer: initial reporting of RTOG 0246. Int J Radiat Oncol Biol Phys 2012;82:1967-72. [Crossref] [PubMed]
- Swisher SG, Moughan J, Komaki RU, et al. Final Results of NRG Oncology RTOG 0246: An Organ-Preserving Selective Resection Strategy in Esophageal Cancer Patients Treated with Definitive Chemoradiation. J Thorac Oncol 2017;12:368-74. [Crossref] [PubMed]
- Piessen G, Messager M, Mirabel X, et al. Is There a Role for Surgery for Patients with a Complete Clinical Response after Chemoradiation for Esophageal Cancer? An Intention-to-Treat Case-Control Study. Ann Surg 2013;258:793-9; discussion 799-800. [Crossref] [PubMed]
- Finks JF, Osborne NH, Birkmeyer JD. Trends in Hospital Volume and Operative Mortality for High-Risk Surgery. N Engl J Med 2011;364:2128-37. [Crossref] [PubMed]
- Naik KB, Liu Y, Goodman M, et al. Concurrent chemoradiotherapy with or without surgery for patients with resectable esophageal cancer: An analysis of the National Cancer Data Base. Cancer 2017;123:3476-85. [Crossref] [PubMed]
- Furlong H, Bass G, Breathnach O, et al. Targeting therapy for esophageal cancer in patients aged 70 and over. J Geriatr Oncol 2013;4:107-13. [Crossref] [PubMed]
- Scarpa M, Valente S, Alfieri R, et al. Systematic review of health-related quality of life after esophagectomy for esophageal cancer. World J Gastroenterol 2011;17:4660. [Crossref] [PubMed]
- Darling GE. Quality of Life in Patients with Esophageal Cancer. Thorac Surg Clin 2013;23:569-75. [Crossref] [PubMed]