Strategies of nodal staging of the TNM system for esophageal cancer
The UICC/AJCC TNM staging for esophageal cancer (8th edition) will begin in use since 2018 (1). Thirty-three institutions from six continents submitted data of 22,654 esophageal and esophagogastric junction cancer patients (2). The TNM staging system was used worldwide to define the point in the natural history at presentation, facilitate treatment recommendations, allow establishment of prognosis and help scientific reporting and comparison. The N staging in the 8th edition of TNM staging remains unchanged from the 7th edition that based on the number of lymph nodes (LN) involved, except the limited revision of the regional LN map (3).
The N staging of the 8th edition of TNM staging for esophageal cancer
The regional LN map of esophageal cancer is revised in 8th edition of TNM staging (Table 1). The supraclavicular node is omitted in group 1 LN, and the groups 5, 6, 10 with uncommon metastasis are omitted as well. Upper thoracic paraesophageal nodes (8U) are used in replace of group 3 LN. And the groups 1, 2, 4, 9 are subclassified into left (L) and right (R) subgroups, respectively. The role and prognostic predicting of the omitted LNs need further investigation and verification.
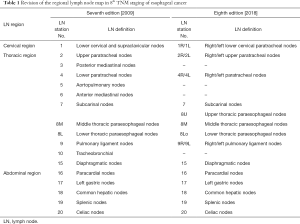
Full table
The 8th edition of Nodal staging is still based on the number of metastatic LN: Nx, LN not to be assessed; N0, noregional LN metastasis; N1, 1–2 regional LN metastasis; N2, 3–6 regional LN metastasis; and N3, ≥7 regional LN metastasis.
There are two patterns of lymphatic spreading of esophagus including penetrating the esophageal wall transversally and flowing longitudinally cephalad (cervical region) and downwards (abdominal region). And the longitudinal lymphatic flow of esophagus is more plentiful than the transverse distribution (4). Lymphatic vessels in the submucosal layer could connect directly to the thoracic duct through the muscularis propria, probably forming the thoracic duct-vascular metastasis of the esophageal cancer (5). The anatomic distribution of lymphatic drainage from the esophagus determines the LN metastasis could vary in the fields of neck, mediastinum and abdomen. About 31.2% of esophageal cancer patients present with 1 field LN involvement, 18.7% with 2 fields, and 2.6% with 3 fields involvement (6). LN metastasis of upper thoracic esophageal cancer usually occurs upwards to upper mediastinal and cervical regions. And the perigastric area is the frequently seen area of LN metastasis from the cancer of distal esophagus (4). Meanwhile, skip metastasis could be present in 20% of patients with esophageal cancer, and a higher incidence of skip LN metastasis could be found in superficial cancer or tumors in the middle/upper esophagus. The skip metastasis was found associated with better survival compared to continuous LN metastasis of esophageal cancer (7).
Evolution of the nodal staging of esophageal cancer
The nodal staging was simply classified into N0 (no LN metastasis) and N1 (with LN metastasis) before the 7th edition of TNM staging [2009] for esophageal cancer. Many reports found that the prognosis of N1 patients according to the 6th edition differed significantly, suggesting that the simple N0/N1 staging couldn’t define the disease progression and predict survival. Kimura et al. early in 1999 found that the number of positive LNs of thoracic esophageal cancer influenced survival. Patients with 4 or more positive LNs had worse prognosis (8). The 5-year survival rate was 90% for patients having no LN metastasis, 52.2% with 1–4 LNs, and 28.9% for 5 or more LNs metastases, respectively (P<0.05) (9). Eloubeidi et al. suggested in 2002 that TNM system for esophageal cancer should consider adding other two significant factors including tumor length and number of metastatic LNs (10).
The revision of N staging in the 7th edition of TNM staging for esophageal cancer was the largest redefinition according to the metastatic LN number (N0–N3). The 7th edition of TNM staging was verified using the data from different centers, demonstrating that the newly refined N staging could better predict the survival of the esophageal cancer patients. Talsma et al. revealed that pT, pN and pM stages could predict overall survival significantly using the 7th edition stratifying compared to the 6th edition (11). As well for the esophageal cancer patients treated with neoadjuvant chemotherapy followed by esophagectomy, the different ypN stages after neoadjuvant treatment based on 7th edition showed significantly different survival probability (ypN0 vs. ypN1, P=0.001; ypN2 vs. ypN3, P=0.004) (12). The report showed that the postoperative three-year overall survival of N0, N1, N2 and N3 stages of Chinese patient with esophageal cancer were 71.8%, 54.4%, 31.6% and 25.0% respectively, and survival curve of each N subgroup separated (P<0.000) (13). Meanwhile the difference of 5-year survival rates among N0, N1, N2 and N3 stages were significantly different (50.0%, 31.5%, 18.7% and 16.7%, P<0.01) (14,15).
The N staging of 7th and 8th edition TNM staging of esophageal cancer was defined according to the number of metastatic LNs, so that the number of harvested LNs at surgery played important role influencing the N staging. Early in the 5th edition, at least 6 LNs were suggested to resect, and at least 12 LNs were suggested in the 7th edition of TNM staging. According to our previous study, if the resected LN number at esophagectomy for cancer was less than six, a nodal downstaging might be classified because of possible unresected occult metastatic LNs (16). Meanwhile, a higher nodal count could favorably influence the operative survival of esophageal cancer (17). Extensive resection of regional LN for esophageal cancer was associated with better prognosis except at the extremes of TisN0 and severely advanced stage (more than 7 metastatic LNs) (18,19). The benefit of extensive lymphadenectomy consisted of obtaining a more accurate N stage and improved long-term survival. In other hand, the extensive lymphadenectomy associated complications, such as recurrent laryngeal nerve injury and lymphatic leak, should be paid attention at surgery. The 8th edition of TNM staging as well recommended the technical requirements at lymphadenectomy including the en bloc resection of LNs and connective tissues, LN counting by the surgeons (1).
The exploration of nodal staging strategy: any more accurate LN index for staging?
Refined N staging based on the number of metastatic LN station
The lymphatic drainage of esophagus involves cervical, mediastinal and abdominal fields resulting in the complicated LN metastasis of esophageal cancer. The metastatic LNs alone in single field are significantly different from the same number of metastatic LNs scattering in two or three fields. Thus, the extent of LN metastasis should not be ignored and might be more important than LN number itself for reflecting the nodal metastasis status. Some disadvantages were found since publication of 7th edition TNM staging of esophageal cancer. Yamasaki suggested that there were no significant survival differences among N2, N3, and M1 subgroups of patients with esophageal cancer (20). Ning et al. further reported that no significant survival differences were found between N2 and N3 subgroups (P=0.231) defined by the 7th edition of TNM system. However, if the staging system was modified based on the metastatic LN filed (N0, no metastatic LN; N1, metastatic LN in 1 field; N2, metastatic LNs in 2 fields; N3, metastatic LNs in more than 2 fields), the survival difference between refined N2 and N3 could be well discriminated (21). Moreover, the LN counting became more important while the 7th edition TNM staging was used. Several metastatic LNs could fused as one mass that was hardly to exactly count the number, in other hand the LN fragmentation at lymphadenectomy may increase the number resulting in up-staging.
Our previous study as well revealed that no significant difference in survival was found for N2 versus N3 subgroups patients based on 7th edition staging. However, when patients were classified based on the extent of metastatic LN (0, 1 filed and 2 fields), significant differences in survival could be observed overall and between each subgroup (22). Further investigation was carried out on our data of esophageal cancer patients in order to define an LN index to reflect metastatic LN extent. We introduced a new system of nodal classification including 4 subgrounds: rN0 (revised N0, no positive LN), rN1 (number of metastatic LN station was 1), rN2 (number of metastatic LN station was 2–3), and rN3 (number of metastatic LN station was ≥4). Survival curves could be significantly distinguished between each subgroups including rN2 versus rN3 (P=0.001) according to our revised station-based nodal staging system (23). Furthermore, the multifactor regression analysis demonstrated that the influence factors of rN staging consisted of tumor invasion depth, differentiation, tumour length, lymphovascular involvement and number of resected LN stations. It’s recommended that more than six LN stations should be resected in order to get accurate rN staging according to Cox’s proportional hazards regression analysis (24).
The revised nodal staging system defined by the station number of metastatic LN could better predict the survival. Meanwhile, it could help to obtain a more reliable and valid clinical-TNM staging (cTNM) by PET or EUS/EBUS needle aspiration to identify the number of involved LN stations, avoiding the difficulty in counting precisely involved LNs that solely depended on surgery.
N staging of the esophagogastric junction cancer
There were still lots of controversies over the staging classification system for esophagogastric junction cancer. The definition of the esophagogastric junction has been revised in 8th edition TNM staging, such that cancers involving it with epicenters no more than 2 cm into the gastric cardia are staged as adenocarcinomas of the esophagus and those with more than 2-cm involvement of the gastric cardia are staged as gastric cancers (3). The TNM classification for adenocarcinoma of esophagogastric junction (AEJ) using the 7th edition TNM staging system presented disadvantage of distinctiveness at each subgroup to predict the postoperative survival of Siewert type II/III AEJ, compared with the gastric cancer TNM staging application (25). Hasegawa found the survival curves between stages II and III of Siewert II/III AEJ were significantly separated in gastric cancer TNM staging (P=0.019), but not in esophageal cancer TNM staging (P=0.204) (26). However, other study showed that it was unnecessary to differentiate between these tumors of adenocarcinoma of the distal esophagus and AEJ, of which the distribution of LN metastases and prognosis were similar (27).
AEJ needs a special LN staging classification taking into account both the positive LN number and site of LN involvement (28). Abdominal nodes were involved commonly in AEJ and mediastinal LN metastases were 46.2%, 29.5% and 9.3% for Siewert type I, II, and III tumors, respectively (29). We also analyzed the lymphadenectomy extent of Siewert type II AEJ using the index of estimated benefit from LN dissection, the 8M, 8L, 16, 17, and G3 had a high therapeutic value and should be resected (30). The N staging of AEJ should not simply depend on esophageal or gastric cancer TNM system. A novel nodal staging system particular for AEJ should be established according to the LN distribution, metastatic LN number and extent.
Lymph node ratio (LNR)
LNR was the ratio of metastatic LN number to total harvested LN number. Five-year disease-specific survival was 30%, 16% and 13% of the patients with an LNR ≤0.2, 0.21–0.5 and >0.5, respectively (P<0.001) (31). The survival difference could be found as well between LNR subgroups of ≤0.2 and >0.2 (32). Hsu et al. reported the prognostic value of the number of negative LNs in esophageal cancer. A higher number of negative LN was associated with better overall survival of esophageal cancer patients after surgery (33). The concept of negative LN number was to some extent in accord with LNR.
However, the influencing factor of LNR was accurately counting metastatic LN number (numerator) and number of resected LN (denominator), which was influenced by the surgeon’s experience and pathologic review. Rice et al. illustrated that with LNR of 0.25, patients with one N+ of four resected, 4 of 16, and 10 of 40 couldn’t have similar survival. Conversely, patients with truly one metastatic LN of four resected (LNR =0.25), 1 of 16 (LNR =0.063), and 1 of 40 (LNR =0.025) didn’t either have significantly different survival (34). Wei et al. found that LNR has better prognostic value than N staging for esophageal cancer if the total number of LN removed was less than 12, which was the contradiction to the lymphadenectomy requirement (35). Fu et al. evaluated a revised nodal category based on the value of the LN station ratio (SR, metastatic LN stations/examined LN stations) in esophageal squamous cell carcinoma patients. The SR category demonstrated superior prognostic ability relative to the AJCC pN category in ESCC patients (36). SR could partially avoid the limitation and disadvantage of LNR mentioned above but needs further investigation.
Lymphovascular invasion (LVI)
LVI distinguished the biologic characteristics of early stage esophageal cancer as the potential key step toward LN metastasis. The 5-year survival rate for patients with T1b esophageal cancers without LVI was 77%, which was similar to the rate of T1a cancer (90%; P=0.08), but it was higher than the survival rate of T1b cancer with LVI (27%; P=0.006) (37). LN metastasis was also frequently seen with LVI as tumor invasion deeper than the muscularis mucosa (38). The high lymphatic microvessel density had an increased risk of LVI development and LN metastasis (39). The presence of LVI and/or LN metastasis could result in worse 5-year survival (37%) compared with the lack of LVI and/or LN metastasis (88%; P<0.001) (37). LVI in LN metastasis was considered as a significant independent prognostic factor in disease progression of esophageal cancer (40).
We have conducted a study with a larger cohort (n=347) and focused on the predictive value of LVI in the primary tumor. Our study highlighted that LVI has a significant impact on the overall survival of patients with resected esophageal squamous cell carcinoma (41). Theoretically, the LVI was a phase of LN metastasis. Thus, there is considerable effect of interaction between LVI and LN metastasis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Amin MB. AJCC cancer staging manual (8th edition). Chicago: Springer, 2017:185-202.
- Rice TW, Apperson-Hansen C, DiPaola LM, et al. Worldwide Esophageal Cancer Collaboration. clinical staging data. Dis Esophagus 2016;29:707-14. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017;12:36-42.
- Ji X, Cai J, Chen Y, et al. Lymphatic spreading and lymphadenectomy for esophageal carcinoma. World J Gastrointest Surg 2016;8:90-4. [Crossref] [PubMed]
- Kuge K, Murakami G, Mizobuchi S, et al. Submucosal territory of the direct lymphatic drainage system to the thoracic duct in the human esophagus. J Thorac Cardiovasc Surg 2003;125:1343-9. [Crossref] [PubMed]
- Li B, Chen H, Xiang J, et al. Pattern of lymphatic spread in thoracic esophageal squamous cell carcinoma: A single-institution experience. J Thorac Cardiovasc Surg 2012;144:778-85; discussion 785-6. [Crossref] [PubMed]
- Prenzel KL, Bollschweiler E, Schröder W, et al. Prognostic relevance of skip metastases in esophageal cancer. Ann Thorac Surg 2010;90:1662-7. [Crossref] [PubMed]
- Kimura H, Konishi K, Arakawa H, et al. Number of lymph node metastases influences survival in patients with thoracic esophageal carcinoma: therapeutic value of radiation treatment for recurrence. Dis Esophagus 1999;12:205-8. [Crossref] [PubMed]
- Tachibana M, Yoshimura H, Kinugasa S, et al. Clinicopathologic factors correlated with number of metastatic lymph nodes in oesophageal cancer. Dig Liver Dis 2001;33:534-8. [Crossref] [PubMed]
- Eloubeidi MA, Desmond R, Arguedas MR, et al. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer 2002;95:1434-43. [Crossref] [PubMed]
- Talsma K, van Hagen P, Grotenhuis BA, et al. Comparison of the 6th and 7th Editions of the UICC-AJCC TNM Classification for Esophageal Cancer. Ann Surg Oncol 2012;19:2142-8.
- Mehta SP, Jose P, Mirza A, et al. Comparison of the prognostic value of the 6th and 7th editions of the Union for International Cancer Control TNM staging system in patients with lower esophageal cancer undergoing neoadjuvant chemotherapy followed by surgery. Dis Esophagus 2013;26:182-8.
- He J, Wang JJ. Survival comparasion after esophagectomy for cancer based on sixth and seventh UICC-AJCC TNM staging: cohort of 400 patients. Tumor 2013;33:164-70.
- Lü F, Xue Q, Shao K, et al. Preliminary experience of clinical applications of the 7th UICC-AJCC TNM staging system of esophageal carcinoma. Zhonghua Zhong Liu Za Zhi 2012;34:461-4. [PubMed]
- Wang J, Wu N, Zheng QF, et al. Evaluation of the 7th edition of the TNM classification in patients with resected esophageal squamous cell carcinoma. World J Gastroenterol 2014;20:18397-403.
- Hu Y, Hu C, Zhang H, et al. How does the number of resected lymph nodes influence TNM staging and prognosis for esophageal carcinoma? Ann Surg Oncol 2010;17:784-90. [Crossref] [PubMed]
- Altorki NK, Zhou XK, Stiles B, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 2008;248:221-6. [Crossref] [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Yamasaki M, Miyata H, Miyazaki Y, et al. Evaluation of the nodal status in the 7th edition of the UICC-TNM classification for esophageal squamous cell carcinoma: proposed modifications for improved survival stratification: impact of lymph node metastases on overall survival after esophagectomy. Ann Surg Oncol 2014;21:2850-6.
- Ning ZH, Wang ZG, Chen J, et al. Proposed Modification of Nodal Staging as an Alternative to the Seventh Edition of the American Joint Committee on Cancer Tumor-Node-Metastasis Staging System Improves the Prognostic Prediction in the Resected Esophageal Squamous-Cell Carcinoma. J Thorac Oncol 2015;10:1091-8.
- Xu QR, Zhuge XP, Zhang HL, et al. The N-classification for esophageal cancer staging: should it be based on number, distance, or extent of the lymph node metastasis? World J Surg 2011;35:1303-10. [Crossref] [PubMed]
- Peng J, Wang WP, Dong T, et al. Refining the Nodal Staging for Esophageal Squamous Cell Carcinoma Based on Lymph Node Stations. Ann Thorac Surg 2016;101:280-6. [Crossref] [PubMed]
- Peng J, Wang WP, Yuan Y, et al. Adequate lymphadenectomy in patients with oesophageal squamous cell carcinoma: resecting the minimal number of lymph node stations. Eur J Cardiothorac Surg 2016;49:e141-6. [Crossref] [PubMed]
- Suh YS, Han DS, Kong SH, et al. Should adenocarcinoma of the esophagogastric junction be classified as esophageal cancer? A comparative analysis according to the seventh AJCC TNM classification. Ann Surg 2012;255:908-15. [Crossref] [PubMed]
- Hasegawa S, Yoshikawa T, Aoyama T, et al. Esophagus or stomach? The seventh TNM classification for Siewert type II/III junctional adenocarcinoma. Ann Surg Oncol 2013;20:773-9. [Crossref] [PubMed]
- Leers JM, DeMeester SR, Chan N, et al. Clinical characteristics, biologic behavior, and survival after esophagectomy are similar for adenocarcinoma of the gastroesophageal junction and the distal esophagus. J Thorac Cardiovasc Surg 2009;138:594-602; discussion 601-2. [Crossref] [PubMed]
- de Manzoni G, Pedrazzani C, Verlato G, et al. Comparison of old and new TNM systems for nodal staging in adenocarcinoma of the gastro-oesophageal junction. Br J Surg 2004;91:296-303. [Crossref] [PubMed]
- Pedrazzani C, de Manzoni G, Marrelli D, et al. Lymph node involvement in advanced gastroesophageal junction adenocarcinoma. J Thorac Cardiovasc Surg 2007;134:378-85. [Crossref] [PubMed]
- Peng J, Wang WP, Yuan Y, et al. Optimal Extent of Lymph Node Dissection for Siewert Type II Esophagogastric Junction Adenocarcinoma. Ann Thorac Surg 2015;100:263-9. [Crossref] [PubMed]
- Greenstein AJ, Litle VR, Swanson SJ, et al. Prognostic significance of the number of lymph node metastases in esophageal cancer. J Am Coll Surg 2008;206:239-46. [Crossref] [PubMed]
- Mariette C, Piessen G, Briez N, et al. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg 2008;247:365-71. [Crossref] [PubMed]
- Hsu PK, Huang CS, Wang BY, et al. The prognostic value of the number of negative lymph nodes in esophageal cancer patients after transthoracic resection. Ann Thorac Surg 2013;96:995-1001. [Crossref] [PubMed]
- Rice TW, Blackstone EH. Lymph node ratio: a confounded quotient. Ann Thorac Surg 2013;96:744. [Crossref] [PubMed]
- Wei C, Deng WY, Li N, et al. Lymph Node Ratio as an Alternative to the Number of Metastatic Lymph Nodes for the Prediction of Esophageal Carcinoma Patient Survival. Dig Dis Sci 2015;60:2771-6. [Crossref] [PubMed]
- Fu X, Liu Q, Luo K, et al. Lymph node station ratio: Revised nodal category for resected esophageal squamous cell carcinoma patients. J Surg Oncol 2017;116:939-46. [Crossref] [PubMed]
- Cen P, Hofstetter WL, Correa AM, et al. Lymphovascular invasion as a tool to further subclassify T1b esophageal adenocarcinoma. Cancer 2008;112:1020-7. [Crossref] [PubMed]
- Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg 2011;253:271-8. [Crossref] [PubMed]
- Schoppmann SF, Jesch B, Zacherl J, et al. Lymphangiogenesis and lymphovascular invasion diminishes prognosis in esophageal cancer. Surgery 2013;153:526-34. [Crossref] [PubMed]
- Schiefer AI, Schoppmann SF, Birner P. Lymphovascular invasion of tumor cells in lymph node metastases has a negative impact on survival in esophageal cancer. Surgery 2016;160:331-40. [Crossref] [PubMed]
- Yang YS, Wang WP, Chen LQ. The effect of interaction between lymphovascular invasion and lymph node metastasis. Surgery 2017;161:1466-7. [Crossref] [PubMed]


