Models of disuse muscle atrophy: therapeutic implications in critically ill patients
Introduction
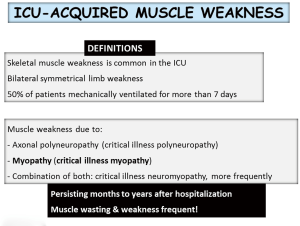
Skeletal muscle weakness is common in the intensive care units (ICU). Approximately 50% of patients under mechanical ventilation for more than 7 days show signs of ICU-acquired muscle weakness (1,2). In these patients, muscle weakness may be the result of axonal polyneuropathy, myopathy or a combination of both, the most common scenario. ICU-acquired muscle weakness may persist for months or years even after hospital discharge, and is frequently associated with muscle wasting (Figure 1). The current review focuses on the underlying biological features that lead to muscle dysfunction and mass loss in critically ill patients. The review is part of a series of reviews that will be published in this issue of the journal, which has been dedicated to the Second International Symposium on Acute Pulmonary Injury Translational Research organized by Hospital Universitario de Getafe, in November 2017. The review will give a short overview of the literature on the most relevant aspects involving the pathophysiology, biological events, experimental models, and potential diagnostic and therapeutic tools currently available for the treatment of ICU-acquired weakness (AW) in clinical settings. The contents described in this review have been presented during the symposium.
The problem and epidemics of ICU-AW
Muscle atrophy and weakness have long been recognized as manifestations of severe illness. In a seminal multicenter study, in which 124 patients were enrolled, it was demonstrated that in the patients who required at least 5 days of mechanical ventilation, ICU-AW diagnosed by MRC exam or handgrip dynamometry was independently associated with greater hospital mortality (3). Handgrip strength was also independently associated with poor hospital outcome, thus implying that it may be used as a simple tool to identify ICU-AW in critically ill patients (3).
The impact of ICU-acquired neuromuscular dysfunction is rather difficult to estimate. In survivors of critical illnesses, the functional disability and impaired quality of life have been related to neuromuscular complaints including persistent pain, contractures, and muscle weakness. Electrophysiological studies have demonstrated the presence of focal compressive mononeuropathy, polyneuropathy, and/or myopathy persisting to months to years after the acute critical illness. In the ICU acute setting, muscle weakness is associated with prolonged mechanical ventilation, failure to separate from the ventilator, delayed discharge from the ICU and hospital, and greater costs. Recognition of neuromuscular dysfunction as an important and potentially long-term manifestation of critical illness that has led to the identification of preventive or therapeutic interventions including glycemic control, measures focused on early mobility, physical and occupational therapy.
Pathophysiological and biological mechanisms
Risk factors in the patients
The commonest risk factors in patients with ICU-acquired muscle weakness are the severity and duration of the systemic inflammatory response, duration of the stay in the ICU and of mechanical ventilation, hyperglycemia, hypoalbuminemia, parenteral nutrition, and administration of corticosteroids and of neuromuscular blocking agents (1,4).
Biological mediators in muscles
Alterations in skeletal muscle morphology have been shown in several models of critical illness and prolonged immobilization. A wide variety of histological features have been found muscle sample preparations obtained from patients with ICU-AW. Loss of thick filaments (myosin), atrophy of the myofibers, necrosis, and regeneration features have been consistently shown in muscle samples during critical illness (2).
In patients with electrophysiological characteristics of critical illness polyneuropathy (CIP), nerve pathology reveals a primarily distal axonal degeneration involving both sensory and motor fibers with no evidence of demyelination or inflammation. Muscle biopsies from these patients exhibit denervation features as well as signs of myopathy. As abovementioned muscle pathology includes acute necrosis, regeneration, type II fiber atrophy, and selective but patchy loss of thick filaments (myosin). The last feature is directly visualized by electron microscopy and it is considered to be a hallmark of critical illness myopathy (CIM), known as “thick filament myopathy”. This observation of myosin loss points towards a potential predominant role of the cellular proteolytic systems in the ICU-AW and experimental models of sepsis (2).
A slow-to-fast fiber type shift, reduced muscle fiber cross-sectional area of the myofibers, alterations in muscle contractility, reduced aerobic capacity and protein synthesis, and the electromechanical properties of the nerve-muscle interface are also relevant features in skeletal muscles of critically ill patients and experimental models (1,5-7). All these biological alterations lead to decreased muscle strength and fatigue resistance in the patients.
The presence of anabolic resistance has also been demonstrated in critically ill patients. The risk factors described in the previous section blunt muscle protein synthesis in the critically ill patient (8-11). This is an important issue in critical illness as insulin or excess dietary amino acid loading had no effect on muscle protein synthesis in the patients (12,13). However, insulin inhibited muscle protein breakdown effectively (12). In keeping with, intensive insulin therapy to control hyperglycemia is associated with a lower number of neuromuscular events related to critical illness in the patients (14).
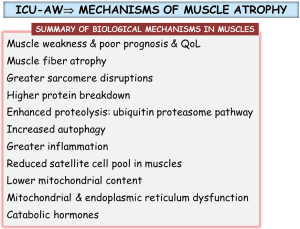
Several studies have also demonstrated the contribution of the atrophy-hypertrophy signaling pathways in the muscles of critically ill patients (15). As such atrogin-1 and caspase-3 expression levels were upregulated and atrophy was present in the diaphragm of mechanically ventilated patients (16). Another investigation also demonstrated atrophy of the rectus femoris of critically ill patients (17). In the blood of critically ill patients, levels of growth and differentiation factor (GDF)-15 were significantly greater while those of muscle-specific microRNAs were lower (17). Sarcomere disruptions along with a significant decrease in the content of both slow- and fast-twitch muscle fibers and increased ubiquitin-proteasome activity were also demonstrated in the diaphragm of critically ill patients (18). In the quadriceps of critically ill patients, the number of satellite cells and mitochondria were reduced whereas autophagy and proteolysis levels were increased in the same muscles (19). Interestingly, after ICU discharge, the quadriceps of patients with persistent muscle atrophy exhibited a lower number of satellite cells (19). Recently, several important studies have also demonstrated the contribution of other biological mediators in the process of ICU-acquired muscle weakness in actual patients such as catabolic hormones, mitochondrial and endoplasmic reticulum dysfunction, and cellular stress (20-22). Moreover, premorbid obesity, but not nutrition, prevented critical illness-induced muscle wasting and weakness in an experimental mouse model (23). A summary of the most relevant biological mediators is provided in Figure 2.
Definitions relevant to the diagnosis
Despite the difficulties to articulate a comprehensive diagnostic nomenclature and classification, an effort has been made to establish the following definitions. First of all, the term ICU-acquired muscle weakness refers to clinically detected weakness in critically ill patients in whom there is no plausible etiology other than critical illness (Figure 1). Patients with ICU-AW and documented polyneuropathy and/or myopathy are classified into three categories: (I) CIP refers to patients with ICU-AW who have electrophysiological evidence of an axonal polyneuropathy; (II) CIM refers to patients with ICU-AW who have electrophysiologically and/or histologically defined myopathy; and (III) critical illness neuromyopathy (CINM) indicates patients who have electrophysiological and/or histological findings of coexisting CIP and CIM (1,2,24).
Weakness affects the extremities and the diaphragm with relative sparing of the cranial nerves such that facial grimace is usually preserved. The finding of generalized weakness is extremely common in critically ill patients and warrants a review of etiologies that not necessarily have always been acquired in the ICU. As some of those other conditions may be treatable, ICU-AW should usually be regarded as a diagnosis of exclusion (1,2,24).
Diagnosis of CIP
CIP is identified in patients who meet the criteria for ICU-AW and who have electrophysiological evidence of a sensorimotor axonal polyneuropathy. Physical examination reveals quadriparesis or quadriplegia, decreased muscle tone, sparing of cranial nerves (grimace in response to noxious stimulus, absence of ptosis or extraocular muscle deficits). If possible, sensory examination reveals a predominantly distal loss of pain, temperature, and vibration sense. Deep tendon reflexes are usually decreased or absent, but normal reflexes do not exclude CIP. Diagnosis of CIP requires differentiation from CIM and from other causes of generalized weakness and other polyneuropathies (1,2,24).
Diagnosis of CIM
CIM is identified in patients who meet the criteria for ICU-AW and who have myopathic features on EMG recorded during voluntary muscle contraction and/or myopathic muscle biopsy. Importantly, physical examination findings are not specific. It is difficult to differentiate CIM from CIP except in fully awake patients in whom the documentation of a new distal sensory loss is suggestive of CIP. However, in patients who are unable to voluntarily contract muscle EMG and/or nerve conduction studies (NCS) differentiates poorly between CIP and CIM, but preserved neuronal action potential amplitudes are suggestive of CIM without coexisting CIP. In patients unable to voluntarily contract, confirmation of CIM is based on muscle biopsy procedures and/or direct muscle stimulation. Pathology of CIM shows: thick filament (myosin) loss, type II fiber atrophy, and necrosis. Besides, increased blood creatine kinase has also been shown in CIM. A differential diagnosis should be conducted between CIM and other conditions such as disuse muscle atrophy, steroid myopathy, cachexia, rhabdomyolysis, and decompensation of primary muscle disorders (1,2,24).
Diagnosis of CINM
CINM is identified when patients meet the criteria for ICU-AW and exhibit features of both CIP and CIM. It seems to be a more prevalent situation than CIP and/or CIM alone.
Diagnosis of prolonged neuromuscular blockade
This condition is identified in critically ill patients who exhibit persistent generalized weakness and dependence on mechanical ventilation after treatment with nondepolarizing neuromuscular blocking agents (NMBAs). Patients with renal and liver failure, metabolic acidosis, and hypermagnesemia are clearly at higher risk. Physical examination shows: flaccid areflexia quadriplegia with involvement of cranial nerves (facial weakness, ptosis, and ophthalmoparesis). The condition is reversible and recovery of motor function is usually observed over a period of 2 to 10 days. The distinction from CIP or CIM is not always clear, especially when weakness is prolonged. Differential diagnosis should also be made with neuromuscular junction diseases such as myasthenia gravis (1,2,24).
Although there is no diagnostic gold standard for ICU-AW or its categories, several measures and tests should be performed in order to identify the problem in the critically ill patient.
Patient assessment & evaluation
Clinical assessment
This is based on the identification of generalized weakness in the appropriate setting, the exclusion of other etiologies of muscle weakness, and the measurement of muscle strength. A careful review of the medical history is required, a careful analysis of the time course of neuromuscular symptoms, especially of those related to the underlying critical illness, and the search for factors potentially associated with ICU-AW such as sepsis, multiple organ failure, mechanical ventilation, hyperglycemia, treatment with corticosteroids and NMBAs, among the most frequent.
Neurological evaluation should include functional domains including consciousness and cognitive functions, cranial nerves, motor and sensory systems, deep tendon reflexes, and coordination. Motor assessment should include the evaluation of muscle strength as well as tone and bulk.
Muscle strength
- Global muscle force is usually explored by means of the Medical Research Council (MRC) score. This is a well validated score that assesses three muscle groups in each of the upper and lower limbs. Each muscle group score ranges from 0 (paralysis) to 5 (normal muscle strength), and the overall score from 0 to 60. Individual MRC scores obtained from predefined muscle groups can be combined in a sum score, yielding a global estimation of motor function. As variability may exist because of differences between examiners and patient collaboration other measures of muscle strength should also be performed.
- Standard handgrip dynamometry (Jamar handgrip dynamometer), which provides a measurement of force on a continuous scale. It has been shown to correlate well with MRC scores and was independently predictive of hospital mortality, suggesting that hand dynamometric assessments might be a surrogate for global strength. Like MRC, dynamometry requires voluntary muscle contraction, which may be compromised by concurrent pain, sedation, delirium, and coma. Differential diagnosis with other conditions should also be made, even in cooperative and alert patients.
Electrophysiology
Electrophysiological methods routinely used to evaluate the peripheral nervous system include NCS, needle EMG, and neuromuscular junction testing. Other parameters of muscle performance such as isometric force assessment have also been used in critically ill patients: dorsiflexor torque to provide peroneal nerve stimulation and ulnar nerve stimulation. Another possibility would be to assess phrenic nerve conduction and needle EMG of the diaphragm, which have been shown to be a valuable tool in the ICU routine electrophysiological testing, especially in patients who fail to liberate from mechanical ventilation.
Ultrasound assessment of muscle thickness
Measurements of muscle thickness in the anterior thigh, forearm, and biceps correlate well with lean body mass. Muscle thickness has also been evaluated in the quadriceps femoris and upper arm in other studies.
Use of potential biomarkers as diagnostic tools
- Increased serum creatine kinase activity has been reported in critically ill patients with acquired myopathy, with a more pronounced elevation in necrotizing myopathy. However, the time course, sensitivity, and specificity of serum creatine kinase have not yet been well established.
- Sarcomeric alpha-actin levels (immunoblotting) have been shown to be increased in the blood of patients with muscle damage in response to exercise.
- Levels of myoglobin can be measured in blood and urine using ELISA procedures.
- Levels of 3-methylhistidine (specific marker of myofibrillar protein degradation) can be measured using a specific assay in plasma and urine. Note: in our center it will be measured only in blood samples.
- The myofilament regulatory protein troponin I can be determined in serum. In skeletal muscles, sTnI exists as two different isoforms: slow sTnI and fast sTnI, produced in slow- and fast-twitch muscle fibers, respectively. The use of specific monoclonal antibodies is crucial in order to detect intact ssTnI and fsTnI in the blood samples.
Potential therapeutic strategies
Understanding the underlying biology of ICU-acquired muscle weakness will help identify pharmacological therapeutic targets in the near future. In this regard, further research is needed in order to design novel therapeutic strategies to restore muscle mass and function. On the other hand, current evidence has shown that the combination of sedation and rehabilitation in the early stages of critical illness improved functional outcome after hospital discharge in critically ill patients (25). Nonetheless, the resources required to rehabilitate patients under mechanical ventilation are very high, thus they may not be available in all ICU settings. Neuromuscular electrical stimulation, however, appears as a very useful rehabilitation approach in critically ill patients as the required resources are significantly lower (26). Importantly, the premorbid condition of the critically ill patient is also another important factor to take into consideration at the time of defining the best therapeutic approach. In fact, it has been recently demonstrated that premorbid obesity in mice exposed to critical illness prevented the animals from muscle wasting and weakness (20).
Conclusions
ICU-acquired muscle weakness is a prevalent condition in patients with critical illness. Several factors and biological mediators have been demonstrated to induce muscle mass loss and weakness in these patients. Novel diagnostic tools should aim to facilitate the early diagnosis of muscle weakness in critically ill patients. Early rehabilitation in combination with nutritional support constitutes the basis of the therapeutic strategies to be implemented in ICU. Moreover, future research will need to shed light on additional cellular processes that could also be targeted pharmacologically. An overview of all these aspects has been provided during the Second International Symposium on Acute Pulmonary Injury Translational Research organized by Hospital Universitario de Getafe (Madrid, Spain) in November 2017.
Acknowledgements
Dr. José Angel Lorente and Dr. Oscar Peñuelas are gratefully acknowledged for inviting me to present the contents covered in this review at the Second International Symposium on Acute Pulmonary Injury Translational Research organized by Hospital Universitario de Getafe (Madrid, Spain) in November 2017.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Puthucheary Z, Harridge S, Hart N. Skeletal muscle dysfunction in critical care: wasting, weakness, and rehabilitation strategies. Crit Care Med 2010;38:S676-82. [Crossref] [PubMed]
- Stevens RD, Marshall SA, Cornblath DR, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med 2009;37:S299-308. [Crossref] [PubMed]
- Ali NA, O'Brien JM Jr, Hoffmann SP, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med 2008;178:261-8. [Crossref] [PubMed]
- Truong AD, Fan E, Brower RG, et al. Bench-to-bedside review: mobilizing patients in the intensive care unit--from pathophysiology to clinical trials. Crit Care 2009;13:216. [Crossref] [PubMed]
- Duchateau J, Hainaut K. Electrical and mechanical changes in immobilized human muscle. J Appl Physiol (1985) 1987;62:2168-73. [PubMed]
- Gibson JN, Halliday D, Morrison WL, et al. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) 1987;72:503-9. [Crossref] [PubMed]
- Seki K, Taniguchi Y, Narusawa M. Alterations in contractile properties of human skeletal muscle induced by joint immobilization. J Physiol 2001;530:521-32. [Crossref] [PubMed]
- Glover EI, Phillips SM, Oates BR, et al. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol 2008;586:6049-61. [Crossref] [PubMed]
- Paddon-Jones D, Sheffield-Moore M, Cree MG, et al. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab 2006;91:4836-41. [Crossref] [PubMed]
- Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol (1985) 2009;107:645-54. [PubMed]
- Rennie MJ. Muscle protein turnover and the wasting due to injury and disease. Br Med Bull 1985;41:257-64. [Crossref] [PubMed]
- Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422-4. [Crossref] [PubMed]
- Volpi E, Mittendorfer B, Rasmussen BB, et al. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 2000;85:4481-90. [PubMed]
- Hermans G, Wilmer A, Meersseman W, et al. Impact of intensive insulin therapy on neuromuscular complications and ventilator dependency in the medical intensive care unit. Am J Respir Crit Care Med 2007;175:480-9. [Crossref] [PubMed]
- Batt J, dos Santos CC, Cameron JI, et al. Intensive care unit-acquired weakness: clinical phenotypes and molecular mechanisms. Am J Respir Crit Care Med 2013;187:238-46. [Crossref] [PubMed]
- Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008;358:1327-35. [Crossref] [PubMed]
- Bloch SA, Lee JY, Syburra T, et al. Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs. Thorax 2015;70:219-28. [Crossref] [PubMed]
- Hooijman PE, Beishuizen A, Witt CC, et al. Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients. Am J Respir Crit Care Med 2015;191:1126-38. [Crossref] [PubMed]
- Dos Santos C, Hussain SN, Mathur S, et al. Mechanisms of Chronic Muscle Wasting and Dysfunction after an Intensive Care Unit Stay. A Pilot Study. Am J Respir Crit Care Med 2016;194:821-30. [Crossref] [PubMed]
- Goossens C, Marques MB, Derde S, et al. Premorbid obesity, but not nutrition, prevents critical illness-induced muscle wasting and weakness. J Cachexia Sarcopenia Muscle 2017;8:89-101. [Crossref] [PubMed]
- Thiessen SE, Derde S, Derese I, et al. Role of Glucagon in Catabolism and Muscle Wasting of Critical Illness and Modulation by Nutrition. Am J Respir Crit Care Med 2017;196:1131-43. [Crossref] [PubMed]
- Thiessen SE, Van den Berghe G, Vanhorebeek I. Mitochondrial and endoplasmic reticulum dysfunction and related defense mechanisms in critical illness-induced multiple organ failure. Biochim Biophys Acta 2017;1863:2534-45. [Crossref] [PubMed]
- Thiessen SE, Vanhorebeek I, Derese I, et al. FGF21 Response to Critical Illness: Effect of Blood Glucose Control and Relation With Cellular Stress and Survival. J Clin Endocrinol Metab 2015;100:E1319-27. [Crossref] [PubMed]
- Fan E, Cheek F, Chlan L, et al. An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med 2014;190:1437-46. [Crossref] [PubMed]
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874-82. [Crossref] [PubMed]
- Gibson JN, Smith K, Rennie MJ. Prevention of disuse muscle atrophy by means of electrical stimulation: maintenance of protein synthesis. Lancet 1988;2:767-70. [Crossref] [PubMed]


