Current therapeutic landscape for advanced gastroesophageal cancers
Introduction
Gastric and esophageal cancers are respectively the 3rd and the 6th most common cause of cancer-related death worldwide (1), essentially because the majority patients are diagnosed in advanced, stage, resulting in a five-year survival rate of 18.8% and 30.6% for esophageal cancer and gastric cancer (GC), respectively (2). Systemic chemotherapy remains the backbone therapy for advanced gastroesophageal cancer but its efficacy is disappointing, with median overall survival (OS) not exceeding 12 months and often <10 months. The anti-HER2 antibody trastuzumab was the first example of modestly successful therapy based on enrichment (3). Anti-angiogenic agents have also produced modest benefit but without patient selection in the second (4) and third-line (5) settings. Immune system modulating agents are actively being studied in these diseases and expectations are high as one phase III trial in the 3rd and later line has produced survival advantage in Asian patients (6). Other therapeutic targets have emerged such as claudin18.2 or STAT3. Fortunately, the clinical trial infrastructure for gastroesophageal cancer research is strong and many trials can be completed in a timely manner.
HER2-positive tumors
Trastuzumab
The epidermal growth factor receptor (EGFR) family of genes is involved in human carcinogenesis, by stimulating several pathways leading to increased proliferation and migration, as well as impaired differentiation and apoptosis (7). Each receptor consists of an extracellular domain, an intracellular domain with kinase activity and a short, lipophilic, transmembrane domain (8). EGFR (receptor I) is activated by ligand binding and induces downstream signaling that involves the Ras/Raf/mitogen-activated protein kinase and phosphatidylinositol-3 kinase/protein kinase-B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathways (9). The HER2-receptor (receptor II) has no known ligand (10). The HER2-receptor can be activated either by homodimerization or heterodimerization with other members of the EGFR family. Overexpression of the HER2-receptor is often associated with cell tumorigenesis and cell proliferation. In a meta-analysis conducted in 17,494 GC patients, HER2 positive rate was 19.1%, even if it seemed slightly lower in Europe than in Asia (respectively 16.9% vs. 19.5%) (11). In this study, it was associated with a poorer prognosis, with a relative risk (RR) of death of 1.47 [95% confidence interval (CI), 1.09–1.98]. The College of American Pathologists, the American Society for Clinical Pathology, and the American Society of Clinical Oncology recently published guidelines for HER2 testing in gastroesophageal adenocarcinoma (ADK) (12). In summary, pathologists should first perform immunohistochemistry (IHC). In case of HER2 positivity (3+) or negativity (0 or 1+), no further test is required. If the IHC results are equivocal (2+), an in situ hybridization method is warranted, and a ratio of HER2 signal to CEP17 (centromere) of 2.0 or greater is considered positive. An increase in gene copy number in the absence of at least 2+ staining by IHC is considered a negative result (HER2 negative).
Trastuzumab is a monoclonal antibody that binds to the extracellular domain of the HER2 receptor leading to antibody-dependent cellular cytotoxicity, inhibition of downstream HER2 signaling (13). In 2010, Bang et al. reported a phase III study evaluating trastuzumab in combination with chemotherapy versus chemotherapy alone for patients with HER2-positive advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma (ToGA trial) (3) (Table 1). The median OS was 13.8 months for the experimental arm compared to 11.1 months for the control arm [hazard ratio (HR) =0.74; 95% CI, 0.60–0.91]. Overall response rate (ORR) was also significantly increased in the trastuzumab arm (47% vs. 35%, P=0.0017). Patients who had tumor with either 3+ overexpression by IHC or IHC 2+ with gene amplification derived more benefit than other subgroups. However, the US FDA analyzed data with a longer follow up and found that initial median OS and HR differences reduced considerably (29). In the ToGA trial, 5% of the patients in the trastuzumab arm experienced a ≥10% drop in left ventricular ejection function (LVEF) to an absolute value <50%. This was consistent with the incidence rate of LVEF decrease in breast cancer patients treated with trastuzumab, which was 7.5% in a meta-analysis published in 2011 (30).
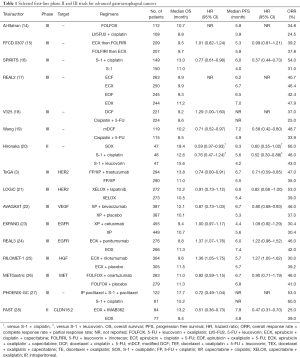
Full table
Due to a more favorable safety profile without efficacy compromise, substitution of cisplatin by oxaliplatin is a desirable in most patients (31). To date, two single-arm phase II studies have combined trastuzumab, oxaliplatin and capecitabine in patients with HER2-positive advanced gastric cancer (AGC) (32,33). The results were similar to initial ToGA results (32,33). Pre-clinical studies showed synergism between oxaliplatin and trastuzumab due to a downregulation of the excision repair cross-complementation group 1 (ERCC1) protein (34).
S-1 is an oral anticancer drug that combines tegafur, a pro-drug of 5-fluorouracil (5-FU), with two modulators (gimeracil and oteracil) (35), which seems to have a better safety profile than 5-FU or capecitabine (36). Japanese phase III trials showed that S-1 was non-inferior to 5-FU and that 5-weekly S-1 plus cisplatin regimen was superior to S-1 alone in AGC patients (16,37). S-1 plus cisplatin with trastuzumab has also been reported with reasonable results in non-randomized studies (38-40). S-1 in combination is a standard first-line treatment of AGC in Japan and it is also approved by the EMA but it is investigational in the USA.
The maintenance of anti-HER2 is often used in breast cancer patients without the lack of strong evidence (41,42). Data in AGC are scarce (43), but a randomized phase II trial is currently evaluating the issue of maintenance in non-progressive patients (NCT02678182) (Table 2). In metastatic colorectal cancer, continuation of anti-angiogenic therapy in second-line despite progression is a standard-of-care (44). To our knowledge, no randomized-controlled trial (RCT) assessed this concept in AGC. However, in a retrospective study conducted in 43 patients treated at the MD Anderson Cancer Center, continuation of trastuzumab beyond disease progression was associated to a median progression free survival (PFS) of 5 months and a median OS of 11 months (45). In another French retrospective multicenter study, continuation (n=39) versus discontinuation (n=65) of trastuzumab beyond progression was significantly associated with an increase on PFS (4.4 vs. 2.3 months; P=0.002) and OS (12.6 vs. 6.1 months; P=0.001) (46). Finally, a Chinese multicenter prospective observational cohort study showed that continuation of trastuzumab beyond progression after first line-therapy improved PFS but not OS (47). In previously treated and progressive HER2-positive tumor patients, the association of trastuzumab, MK-2206 (Akt-inhibitor) and paclitaxel showed significant clinical activity in phase I studies despite prior HER2-directed therapy (48,49). However, further evaluation is needed for considering trastuzumab continuation beyond progression in our daily practice.
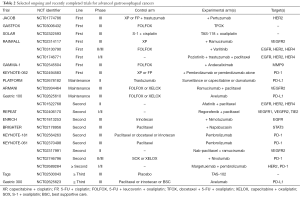
Full table
In HER2-positive AGC patients who did not receive trastuzumab in first line, the latter could be considered in the second-line. No phase III study assessed this issue, but a Japanese phase II study was recently published (50). Forty-seven patients with unresectable or recurrent gastric ADK, previously treated (without trastuzumab or taxane), received paclitaxel and trastuzumab. ORR was 37%, median PFS and OS were respectively 5.1 and 17.1 months. Safety profile was not different compared to the first-line setting.
T-DM1
T-DM1 is an antibody-drug conjugate of trastuzumab and emtansine (DM1), a microtubule inhibitor. This component delivers cytotoxic drugs directly to cancer cells, and demonstrated promising anti-tumor effect in preclinical models (51). In the advanced breast cancer setting, T-DM1 has been associated with significant efficacy and minimal toxicity even in heavily pretreated patients (52). GATSBY trial was a randomized phase II/III study evaluating T-DM1 vs. taxane (docetaxel or paclitaxel) in patients with HER2-positive AGC who progressed during or after first-line therapy (53) (Table 3). Among the 415 included patients, nearly 80% had previously received anti-HER2 therapy. Median OS (primary endpoint) was not improved with T-DM1 compared to taxane treatment (respectively 7.9 vs. 8.6 months; P=0.86). ORRs were also similar, around 20% in both arms. Incidence of grade 3 or more adverse events (mainly hematologic toxicity) was slightly lower in the experimental group compared to the control group (60% and 70% respectively). As a consequence, T-DM1 cannot be considered as a second-line treatment option for patients with HER2-positive AGC.
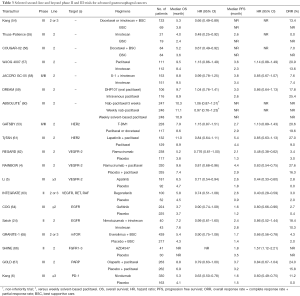
Full table
Lapatinib
Lapatinib is a tyrosine kinase inhibitor (TKI) targeting intracellular domain of HER2 and EGFR, and prevents activation of PI3K and Ras pathways. Efficacy was suggested in HER2-positive GC animal models (68), but the activity in human studies was modest (69). In the LOGiC phase III trial, lapatinib was evaluated with capecitabine and oxaliplatin in treatment-naive patients with advanced HER2-positive tumors (21). Median OS (primary endpoint) and PFS were not significantly improved with lapatinib, compared to the control arm.
In the TyTAN phase III study, 261 Asian patients with advanced HER2-FISH amplified GC, progressive after a first-line therapy, were randomly assigned to receive lapatinib 1,500 mg/day plus paclitaxel 80 mg/m2/week or paclitaxel alone (61). Almost all patients were previously treated with trastuzumab (94%). Median OS was not significantly improved in the lapatinib/paclitaxel group compared to the paclitaxel group (11.0 vs. 8.9 months; P=0.104). As a consequence, this study was closed prematurely for futility. Similar to the first-line setting, lapatinib should not be prescribed in patients with AGC outside clinical trials.
Pertuzumab
Pertuzumab is a recombinant, humanized, monoclonal antibody that targets HER2. It binds to the dimerization domain of HER2 (70). Pertuzumab with trastuzumab have significant antitumor activity in HER2-positive human GC xenograft models (71). The combination of pertuzumab and trastuzumab is effective for breast cancer patients. A phase III trial evaluating the value of this combination with chemotherapy in the first-line treatment in patients with HER2-positive advanced gastroesophageal cancer has completed accrual and results are pending (NCT01774786).
Pan-HER TKIs
Afatinib is an oral irreversible pan-HER TKI targeting HER1 (EGFR), HER2 and HER4 (72). It demonstrated antitumor activity in a HER2-positive xenograft mouse model (73). In a phase II study enrolling 20 patients with advanced HER2-positive (IHC 3+ or FISH amplified) esophagogastric ADK, refractory to trastuzumab, disease stabilization rate was 42% at 4 months (74). Tolerance was acceptable, with mainly grade 1 or 2 skin and digestive toxicities. A phase II study is currently evaluating afatinib combined to paclitaxel in HER2-positive esophago GC patients, progressive after a first-line trastuzumab-based therapy (NCT01522768).
Dacomitinib is another oral pan-HER TKI, which irreversibly inhibits HER1, HER2 and HER4, but also prevents HER1/HER2, HER2/HER3, and HER3/HER4 heterodimerization (75). In a small phase II study conducted in 27 previously treated patients with HER-2 positive GC, the disease control rate was 40.7%, with a good safety profile (76). To our knowledge, no other study evaluating this drug is ongoing.
New anti-HER antibodies
Margetuximab is a monoclonal antibody derived from 4D5, the parent antibody of trastuzumab. It binds the same epitope of HER2 than trastuzumab, with similar affinity, but it engineered with increased affinity for both isoforms of CD16A, a stimulatory receptor present on natural killer cells and macrophages, essential for mediating antibody-dependent cell-mediated cytotoxicity (77). Results of a phase I study evaluating margetuximab in HER-2 positive advanced solid tumors were recently published (78). Twenty patients had a gastroesophageal cancer and the median number of prior chemotherapy regimens was three. The safety profile was excellent, with less than 5% of grade 3 or more adverse events. Two patients had a partial response. A study of margetuximab in combination with pembrolizumab, an anti-programmed death-1 (PD-1) antibody, is ongoing (NCT02689284). MM-111 is an antibody inhibiting heregulin-activated HER3 signaling in HER2+ tumors (79). A randomized phase II study of paclitaxel and trastuzumab ± MM-111 has recently been completed in previously treated patients with advanced HER2-positive esophagogastric cancer (NCT01774851). Results are pending. DS-8201a is a HER2-targeting antibody–drug conjugate which delivers cytotoxic chemotherapy (DXd, a novel topoisomerase I inhibitor) directly to cancer cells. In trastuzumab pretreated AGC patients, DS-8201a was associated with an ORR of 38%, with an acceptable safety profile (80). The phase II is underway (NCT02564900). Finally, ZW25 is a bispecific antibody that can simultaneously bind two non-overlapping epitopes of HER2, resulting in dual HER2 signal blockade. Consequences could be an increased cytotoxicity, an enhanced antibody internalization and HER2 downregulation and an enhanced blockade of ligand-dependent/independent tumor growth. A phase I study is currently recruiting patients with advanced HER2-expressing cancers (NCT02892123).
In summary, the association of trastuzumab with chemotherapy based on platinum components and 5-FU is the standard-of-care in the first-line setting of patients with HER2-positive advanced gastroesophageal cancer. In case of progression after a first-line treatment without trastuzumab, the latter can be used as a second-line therapy, combined with chemotherapy. All prescribed second-line treatments for HER2-negative patients can also be suitable for patients with HER2-positive tumors. Trastuzumab beyond progression and maintenance treatment with trastuzumab are two concepts which warrant further evaluation.
HER2-negative tumors
Chemotherapy
Platinum-based chemotherapies were evaluated first, with median OS between 8 and 11 months (14,17,81,82). Combination of 5-FU and irinotecan is also feasible, with similar efficacy and a better safety profile than anthracycline-containing triplet epirubicin, cisplatin, and capecitabine (ECX) (15). However, in various randomized trials, irinotecan has never produced OS advantage, therefore, irinotecan-based combinations are not recommended in the first line setting. In a recent and well-conducted network meta-analysis, increased efficacy was demonstrated for fluoropyrimidine non-cisplatin doublets over cisplatin doublets, with respective HR for death of 0.85 (95% CI, 0.71–0.99) and 0.83 (95% CI, 0.71–0.98) for 5-FU/irinotecan (FI) and 5-FU/oxaliplatin (FOX) (18). Moreover, PFS was significantly improved with FOX compared to 5-FU/cisplatin (HR =0.82; 95% CI, 0.66–0.99). No difference was observed between FI and FOX concerning OS or PFS. The 2017 NCCN guidelines stated that two-drug cytotoxic regimens should be preferred to triplets due to a lower toxicity (31). In the 2016 ESMO guidelines, doublet or triplet platinum/fluoropyrimidine combinations were both recommended, but authors indicated that it remained controversy regarding the utility of triplet regimens (83).
However, triplets containing taxane such as DCF (docetaxel/5-FU/cisplatin) are also an evidence-based treatment choice for first-line chemotherapy, but these regimens are associated with safety concerns (19,84,85). The most promising chemotherapy triplet associates docetaxel, 5-FU and oxaliplatin (DFOX or TFOX or FLOT). Interesting results were recently reported in a perioperative setting (86). According to the network meta-analysis of Ter Veer et al., PFS was slightly improved with TFOX regimen compared to FI and FOX, but OS was similar (18). However, hematologic and digestive toxicity rates were increased with TFOX versus FOX. A phase III study comparing TFOX and FOLFOX in naïve patients with AGC is ongoing (NCT03006432). S-1 could also be an alternative to conventional 5-FU (20). Peritoneal dissemination is common in case of AGC, and it is associated to a poor survival. However, combined to intravenous paclitaxel and S-1, intraperitoneal paclitaxel does not significantly improve OS compared to S-1 plus cisplatin (27).
Second line therapies (and third line therapies) seem to contribute to patient survival in a minor way and should be considered when patient’s general condition is reasonable. According to a recent meta-analysis, both taxane and irinotecan as single agents showed significantly prolong survival compared to best supportive care (BSC), without difference between these two regimens (87). It confirmed data issued from phase III trials (54-57). Addition of S-1 to irinotecan does not improve survival (58).
In the DREAM study, oral paclitaxel (DHP107) was non-inferior to intravenous paclitaxel in terms of PFS, with a similar safety profile (59). In the ABSOLUTE phase III trial, nab-paclitaxel was non-inferior to paclitaxel in terms of OS (60), but due to the additional cost, the former will not replace the latter. TAS-102 is a novel oral nucleoside antitumor agent containing trifluridine and tipiracil hydrochloride, which prevents the degradation of trifluridine. It showed interesting results in a phase II study (88), justifying third-line phase III study (NCT02500043).
Targeted therapies
Vascular endothelial growth factor (VEGF) is overexpressed in 30% to 60% of esophageal cancers and this is associated with an increased risk of recurrence, distant metastasis and death (89). However, targeted therapies against VEGF do not seem relevant in the first-line treatment of AGC (22,90,91), contrary to the second-line setting. After showing efficacy as a monotherapy (62), ramucirumab became a standard-of-care in association with paclitaxel in previously treated patients with advanced gastric or GEJ ADK (4). After progression with two or more lines of chemotherapy, data were lacking. Apatinib is a small-molecule TKI that highly selectively binds to and strongly inhibits VEGFR-2, with a decrease in VEGF-mediated endothelial cell migration, proliferation, and tumor microvascular density. In 2016, a placebo-controlled randomized study provided the first robust evidence for a third-line therapy based on apatinib in advanced gastric and GEJ tumors (5). Finally, regorafenib is another oral multitarget TKI which showed efficacy in chemorefractory advanced colorectal cancer and hepatocellular carcinoma. In patients with AGC, progressive after one or more lines of chemotherapy, regorafenib improved median PFS but not OS (63). A phase III study with a similar design in the third-line setting is planned (NCT02773524). Regorafenib is also tested combined with paclitaxel as a second-line treatment (NCT02406170).
Anti-EGFR antibodies are approved for patients with metastatic colorectal cancer without RAS mutations (44). KRAS mutation rate appears very low in upper gastrointestinal malignancies, approximatively 4% in GC (92) and 2% in esophageal cancers (93). However, anti-EGFR therapies in addition to standard chemotherapy failed to demonstrate additional benefit of cetuximab (23,94) or panitumumab (24). Nimotuzumab, another antibody against EGFR, was also non-effective in a phase II randomized trial (95). However, combination of nimotuzumab and irinotecan showed potential efficacy as second-line therapy in patients with AGC overexpressing EGFR (EGFR 2+/3+ in IHC) based on improved response, PFS, and OS (96). A phase III trial is ongoing in this subgroup of patients (NCT01813253). Erlotinib, an EGFR oral TKI, showed contrasted results in advanced esophagogastric cancer (97,98) whereby no further investigation is ongoing on this molecule. Gefitinib, an oral EGFR TKI, also failed to improve OS (64).
Mesenchymal epidermal transition (c-MET) is a proto-oncogene coding for a hepatocyte growth factor (HGF) receptor. It is overexpressed in 40% of GC and associated with worse OS (99). Despite encouraging results in phase II studies, two antibodies targeting HGF or MET, rilotumumab and onartuzumab, did not improve OS compared to chemotherapy alone in patients with advanced MET-positive untreated gastric or GEJ cancer (25,26).
As mentioned above, research on targeted therapies in advanced upper gastrointestinal malignancies was very disappointing, with several negative studies. However, very interesting results were reported during the ASCO® 2016 about a new therapeutic class. Claudin18.2 (CLDN18.2) is a tight junction protein expressed by several cancers, but not by normal cells. In the phase II FAST study, patients with advanced/recurrent gastric and GEJ cancer were randomized to first-line EOX (epirubicin, oxaliplatin, capecitabine) with or without IMAB362, a chimeric IgG1 monoclonal antibody that mediates specific killing of CLDN18.2-positive cancer cells (28). IMAB362 (or claudiximab) stimulates cellular and soluble immune effectors that activate antibody-dependent cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC). It can also induce apoptosis and inhibit cell proliferation. When combined with chemotherapy, claudiximab enhances T-cell infiltration and induce pro-inflammatory cytokines (100). In the FAST study, only patients with CLDN18.2-positive tumors were included (CLDN18.2 expression of ≥2+ in ≥40% tumor cells), representing 46% of screened patients. IMAB362 plus EOX improved median PFS (7.9 vs. 4.8 months, P=0.001) and median OS (13.8 vs. 8.4 months, P=0.001) compared to chemotherapy alone. In the subpopulation with very high CLDN18.2 expression (≥2+ intensity in ≥70% tumor cells), efficacy on OS was more pronounced (16.7 vs. 9.0 months, P<0.001). Neutropenia and vomiting episodes were increased in the experimental arm compared to the control arm (respectively 44.2% vs. 33.3% and 58.4% vs. 36.9%). This study provided strong evidence for a positive impact of IMAB362 on survival of patients with CLDN18.2-positive AGC. This new molecule has the potential for changing our practices in a near future. However, a phase III trial is warranted, and to our knowledge, it is still pending.
Other activation pathways, such as mTOR (65), FGFR (66), or PARP (67) were recently targeted, but with frustrating results. STAT3 is a transcription factor regulating activation of key genes involved in cell proliferation, apoptosis, inflammatory response and angiogenesis (101). Activated STAT3 protein expression has been noted in 30–70% of GC, and is correlated to differentiation, stage of disease, lymph node metastases and poor survival (101). Napabucasin is an oral STAT3 inhibitor which was tested in addition to weekly paclitaxel in a phase Ib/II study among 46 patients with advanced, pre-treated (one or more line) gastric or GEJ ADK (102). Safety profile was acceptable with less than 10% of grade 3 or more adverse events. Disease control rate varied between 68% and 83% depending on previous received chemotherapies. The BRIGHTER phase III trial has been terminated after an interim analysis due to lack of efficacy (NCT02178956). Finally, we can cite andecaliximab, a monoclonal antibody inhibitor of matrix metalloproteinase 9, an extracellular enzyme involved in matrix remodeling, tumor growth, and metastasis. After recent encouraging preliminary results (103), those of the phase III in combination with FOLFOX as first-line treatment has completed accrual (NCT02545504).
In conclusion, in patients with HER2-negative AGC, a platinum-based doublet (preferably FOX) is recommended in the first-line setting. Three-drug cytotoxic regimens should be reserved for medically fit patients with good performance status, due to higher toxicity. In such case, TFOX regimen seems to be the most promising triplet protocol. If available, S-1 can replace 5-FU. After progression, second-line regimen of choice combines ramucirumab and paclitaxel, but taxane or irinotecan monotherapy protocols are also relevant options. Apatinib should be considered as a standard-of-care in the third-line setting, however, it has been approved only in China.
Immune checkpoint inhibitors
Since 2011, immune checkpoint inhibitors disrupted the prognosis of metastatic melanoma (104) and lung cancer (105). However, results were more contrasted in gastrointestinal malignancies. PD-1 is a negative co-stimulatory receptor mainly expressed on activated T cells, which downregulates excessive immune responses by binding to its ligands, PD-L1 and PD-L2 (106). In tumor tissues, binding of PD-1 to PD-L1 inhibits effector T-cell function, leading to suppression of the antitumor immune response and enabling neoplastic growth. Most of studies are consistent about the PD-L1 overexpression rate in upper gastrointestinal malignancies, around 40% (107). It is correlated with poorer outcome (108). Recently, results of the first phase III trial evaluating a PD-1 antibody (nivolumab) in unresectable advanced or recurrent gastric or GEJ patients were reported (6). After two or more previous chemotherapies failure, 493 subjects were randomized to receive nivolumab 3 mg/kg (n=330) or placebo (n=163) every 2 weeks. Primary endpoint was met, with a median OS of 5.3 months in the nivolumab group versus 4.1 months in the control group (P<0.0001). Median PFS was also slightly improved with nivolumab compared to placebo (HR =0.60; 95% CI, 0.49–0.75). No data about the PD-L1 status was reported.
Nivolumab was also tested in combination with ipilimumab, an anti-CTLA4 antibody, in the phase I/II study CheckMate-032 (109). Patients (n=160) were randomized in 3 groups: nivolumab alone (3 mg/kg), nivolumab + ipilimumab (respectively 1 and 3 mg/kg) and nivolumab + ipilimumab (respectively 3 and 1 mg/kg). After 4 cycles, all patients received nivolumab 3 mg/kg/2 weeks until confirmed disease progression or intolerable toxicity. ORR was 12%, 24% and 8% in the first, the second and the third group, respectively, and seemed better in case of PD-L1 expression. Corresponding median OS were 6.2, 6.9 and 4.8 months (not reached in PD-L1+ subgroups). Toxicity was increased with bi-therapy (47% of grade 3 or 4 adverse events and treatment discontinuation due to toxicity in 20% of the cases in the second group). A phase III trial is planned.
Pembrolizumab (anti-PD-1 antibody) is tested alone or in combination in 3 different cohorts in the KEYNOTE-059 study. In cohort 1, patients were progressive after 2 or more chemotherapy lines and received pembrolizumab monotherapy at 200 mg/3 weeks. After encouraging results published in 2016 (ORR of 22% in 39 patients) (110), study was continued in phase II (111). A total of 259 were included (52% in third-line, 48% in 4th-line or more), of whom 57% had a PD-L1-positive tumor. ORR was 11.6% (15.5% if PD-L1+ and 6.4% if PD-L1). Interestingly, 57% of the patients with a MSI tumor were responders compared with only 9% of those with a MSS tumor. Almost one patient out of four was alive at one year. Rate of grade 3 or 4 adverse events was only 4.6% (fatigue, anemia, diarrhea, skin rash), confirming the excellent safety profile of immunotherapy. Cohort 2 included treatment-naïve patients with HER2-negative gastric or GEJ ADK, who received combination of pembrolizumab (200 mg/3 weeks) and chemotherapy (cisplatin + 5-FU or capecitabine). Preliminary results in 25 patients showed an ORR of 60% and a median PFS of 6.6 months (112). These results suggest that immune checkpoint inhibitors seem more effective in association with conventional chemotherapy and in a first-line setting. Three phase III studies are currently testing pembrolizumab as a monotherapy or in combination with chemotherapy, in first-line (NCT02494583) or second-line (NCT02564263, NCT02370498) treatment.
Finally, avelumab, an anti-PD-L1 antibody, was evaluated in the phase Ib JAVELIN trial in 151 patients with advanced gastric or GEJ cancer, as a first-line maintenance (group 1) or second-line therapy (group 2) (113). ORR was 9.0% and 9.7% in group 1 and 2, respectively. Corresponding median PFS were 12.0 and 6.0 weeks. Two phase III trials are currently evaluating avelumab in metastatic gastric or GEJ ADK, as a third-line treatment (Gastric 300 or NCT02625623) or as a maintenance therapy (Gastric 100 or NCT02625610).
New generation immune-oncology drugs are currently evaluated in preliminary studies, including novel inhibitory compounds (e.g., TIM-3, VISTA, LAG-3, IDO, KIR) and newly developed co-stimulatory antibodies [e.g., CD40, glucocorticoid-induced TNF-R-related protein (GITR), OX40, CD137, ICOS] (114). VISTA (v-domain Ig suppressor of T cell activation), also known as B7-H5, is mainly expressed by regulatory T cells (Tregs) and tumors. It depletes cytokine synthesis and T cell function while inducing forkhead/winged-helix transcription factor box p3 (Foxp3) synthesis. Foxp3 is a key regulator for CD4+ CD25+ Treg cell differentiation and function. These latter can kill allogeneic target cells including activated CD4+ and CD8+ T cells, CD14+ monocytes, and both immature and mature dendritic cells in a perforin-dependent manner (115). Immunosuppression mediated by Tregs is therefore a key facilitator of tumor immune evasion. VISTA expression is about 9% in GC and seems to be associated with PD-L1 expression (116). Blocking both VISTA and PD-L1 could therefore provide stronger stimulation of anti-tumor immunity. After encouraging results in multiple in vivo models, CA-170, a first-in-class oral small-molecule antagonist that selectively targets PD-L1 and VISTA, is currently tested in a phase I study in patients with advanced tumors and lymphomas (NCT02812875). A fully human IgG1 kappa anti-VISTA monoclonal antibody (JNJ-61610588) is also under evaluation (NCT02671955). IDO (indoleaminepyrrole-2,3-dioxygenase-1,2) catalysis oxidative cleavage of tryptophan in the kynurenine pathway, resulting in a decreased tryptophan level which then suppresses T cell proliferation (117). High expression of IDO was independently associated with poor postoperative clinical outcome of patients with GC (118). Ipilimumab leads to IDO-1,2 overexpression and subsequently to an increased PD-1/PD-L1 stimulation, explaining one of the resistance mechanisms to immunotherapy. Research on IDO-1,2 inhibitors is extensive, but no objective response was reported in patients with advanced esophageal cancer treated with indoximod alone (119) or combined with docetaxel (120) in two phase I studies. However, sample size was very small (respectively 1 and 2 patients). Once again, combination of IDO and PD-L1 inhibitors is intensively evaluating, but with modest preliminary results. In a phase Ib study, partial response rate was 9% with the GDC-0919 (IDO1 inhibitor)/atezolizumab (PD-L1 inhibitor) association in 45 pretreated patients with locally advanced or metastatic solid tumors (121). Agonistic antibodies activating immune cells are another attractive alternative. ICOS (inducible T cell co-stimulator or CD278) is mainly expressed on activated T cells and its ligand (B7-H2) on B cells and dendritic cells. Activation of this pathway leads to increased production of cytokines, especially IL-10. ICOS agonists are currently tested alone (GSK3359609) or in combination with nivolumab (NCT02904226) in phase I/II studies for patients with advanced solid tumors, including esophageal cancers. The GITR and its ligand are present on Tregs, CD4+/CD8+ T cells and natural killer cells. This pathway inhibits Tregs, known as major players in downregulation of antitumor immunity (122). Several phase I studies are evaluating agonistic GITR antibodies alone (NCT02628574) or associated to pembrolizumab (NCT02132754).
Finally, individualized vaccine targeting cancer neo-epitope repertoire is another interesting approach. Different techniques are under assessment, but the concept is to select, expand and administer T cells which will selectively target and destruct cancer cells based on tumor-associated antigens (TAAs) recognition. The latter can be selected thanks to patient’s dendritic cells analysis. To date, the only approved vaccine in oncology is sipuleucel-T, indicated for prostate cancer. Whole tumor cells are another source of TAAs, with the advantage of allowing the complete array of TAAs possible to be presented to the immune system. Protein and peptide vaccines are easier to manufacture but they present a smaller spectrum of antigens to the host immune system. HER2-peptide vaccination of patients with metastatic HER2-positive GC is currently testing (NCT02276300). Viral-based vaccines could be interesting vectors for vaccines. The idea is to encode TAAs in an attenuated virus, which will transduce host cells and lead to antigen expression. However, this approach did not provide positive signals in terms of efficacy at this time.
In conclusion, nivolumab alone showed modest efficacy in a third-line RCT, but combination of different types of immune checkpoint inhibitors as well as association of chemotherapy and immunotherapy seem more promising. Finding relevant predictors of efficacy is needed, but PD-L1 positivity in tumor cells showed limits, contrary to MSI status. Recently, Le et al. analyzed efficacy of pembrolizumab in a cohort of 86 patients with MSI+ tumors (76% if digestive cancers, 5 gastroesophageal tumors) (123). ORR was 53%, of which 21% of complete response. After 2-year follow-up, 53% of the patients did not progress and 64% were still alive (median OS and PFS were not reached). Considering that 22% of GC patients are MSI+ (124), great hopes are permitted with immunotherapy for this subgroup.
Conclusions
Treatment of advanced upper gastrointestinal malignancies remains challenging. Several hopes generated by positive phase II studies were not confirmed in phase III trials. We have to learn from past failures, by creating a real personalized medicine. It starts with a better knowledge of molecular profiles in esophageal and GC, as well as anti-tumor immunity, because it seems illusory to find a unique active drug for a majority of patients. Significant improvements were recently made in this domain, especially with the Cancer Genome Atlas (TCGA) network (124,125). Genomic profiling is becoming widely available, but we must avoid a simplistic view in which one tumor with a genomic alteration will be treated successfully with the targeted therapy against this abnormality. Cancers can display multiple simultaneous driver mutations and develop several and complex escape mechanisms. Two ways of research seem essential: the development of robust predictors and the combination of different therapeutic classes for overcoming current drug resistance. Recent preclinical studies showed that overexpression of heregulin, a HER3 ligand, conferred robust resistance to lapatinib and trastuzumab via HER3-Akt pathway activation followed by survivin overexpression (126). In the future, high intra-tumor heregulin level could be used for predicting anti-HER2 therapy resistance and for improving patient selection in clinical studies. In the same way, aberrant V-ATPase activity in lysosomes could also be a potential biomarker for predicting T-DM1 resistance (127). Concerning immunotherapy, tumor PD-L1 expression reveals its limitations for predicting immune checkpoint inhibitor efficacy. Nevertheless, microsatellite instability status (128) and neoantigen load could be stronger predictors (129). Recently, an aggregated score (immunophenoscore) was built, based on the expression of the representative genes or gene sets comprising four categories: MHC molecules, immunomodulators, effector cells and suppressor cells (130). This score showed a predictive value for efficacy of immune checkpoints inhibitors superior to the expression of checkpoints molecules. For overcoming primary resistance to trastuzumab, the addition of the mTOR inhibitor everolimus could be a way of research, as has been demonstrated in breast cancer (131). The novel HER2/CD3 bispecific antibody also showed interesting results in GC patient-derived xenografts (132). Because ORR does not exceed 30% in case of single-agent immunotherapy, it may be more effective to develop combination treatments to improve outcomes. As mentioned above, several studies are currently evaluating combination of different types of immune checkpoint inhibitors or in association with chemotherapy. Recent data were also reported about combination of pembrolizumab and ramucirumab in previously treated patients (133). Despite a prognosis which remains poor, the esophagogastric cancer treatment armamentarium is becoming broader, but finding relevant predictors for efficacy is needed if we want to make the most of these advances.
Acknowledgements
None.
Footnote
Conflicts of Interest: A Lopez has research funding from Roche, has served as consultant for Amgen and received lecture fees from Vifor Pharma; the other authors declare no conflicts of interest.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Kang YK, Satoh T, Ryu MH, et al. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): A double-blinded, randomized, phase III trial. J Clin Oncol 2017;35:2. [Crossref]
- Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol 2001;12 Suppl 1:S3-8. [Crossref] [PubMed]
- Akiyama T, Sudo C, Ogawara H, et al. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science 1986;232:1644-6. [Crossref] [PubMed]
- Okines A, Cunningham D, Chau I. Targeting the human EGFR family in esophagogastric cancer. Nat Rev Clin Oncol 2011;8:492-503. [Crossref] [PubMed]
- Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 2000;19:3159-67. [Crossref] [PubMed]
- Lei YY, Huang JY, Zhao QR, et al. The clinicopathological parameters and prognostic significance of HER2 expression in gastric cancer patients: a meta-analysis of literature. World J Surg Oncol 2017;15:68. [Crossref] [PubMed]
- Bartley AN, Washington MK, Ventura CB, et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Am J Clin Pathol 2016;146:647-69. [Crossref] [PubMed]
- Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007;357:39-51. [Crossref] [PubMed]
- Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 2008;26:1435-42. [Crossref] [PubMed]
- Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Federation Francophone de Cancerologie Digestive, Federation Nationale des Centres de Lutte Contre le Cancer, and Groupe Cooperateur Multidisciplinaire en Oncologie) study. J Clin Oncol 2014;32:3520-6. [Crossref] [PubMed]
- Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215-21. [Crossref] [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Ter Veer E, Haj Mohammad N, van Valkenhoef G, et al. The Efficacy and Safety of First-line Chemotherapy in Advanced Esophagogastric Cancer: A Network Meta-analysis. J Natl Cancer Inst 2016.108. [PubMed]
- Wang J, Xu R, Li J, et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer 2016;19:234-44. [Crossref] [PubMed]
- Hironaka S, Sugimoto N, Yamaguchi K, et al. S-1 plus leucovorin versus S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin in patients with advanced gastric cancer: a randomised, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:99-108. [Crossref] [PubMed]
- Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial. J Clin Oncol 2016;34:443-51. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490-9. [Crossref] [PubMed]
- Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:481-9. [Crossref] [PubMed]
- Cunningham D, Tebbutt NC, Davidenko I, et al. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study. J Clin Oncol 2015;33:abstr 4000.
- Shah MA, Bang YJ, Lordick F, et al. METGastric: A phase III study of onartuzumab plus mFOLFOX6 in patients with metastatic HER2-negative (HER2-) and MET-positive (MET+) adenocarcinoma of the stomach or gastroesophageal junction (GEC). J Clin Oncol 2015;33:abstr 4012.
- Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III study of intraperitoneal paclitaxel plus s-1/paclitaxel compared with s-1/cisplatin in gastric cancer patients with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol 2016;34:abstr 4014.
- Al-Batran SE, Schuler MH, Zvirbule Z, et al. FAST: An international, multicenter, randomized, phase II trial of epirubicin, oxaliplatin, and capecitabine (EOX) with or without IMAB362, a first-in-class anti-CLDN18.2 antibody, as first-line therapy in patients with advanced CLDN18.2+ gastric and gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol 2016;34:abstr LBA4001.
- Highlights of prescribing information. Available online: www.accessdata.fda.gov/drugsatfda_docs/label/2014/103792s5311lbl.pdf
- Chen T, Xu T, Li Y, et al. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: a meta-analysis. Cancer Treat Rev 2011;37:312-20. [Crossref] [PubMed]
- Benson AB 3rd, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:370-98. [Crossref] [PubMed]
- Ryu MH, Yoo C, Kim JG, et al. Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer 2015;51:482-8. [Crossref] [PubMed]
- Gong J, Liu T, Fan Q, et al. Optimal regimen of trastuzumab in combination with oxaliplatin/ capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer 2016;16:68. [Crossref] [PubMed]
- Ding X, Qu X, Fan Y, et al. Trastuzumab and oxaliplatin exhibit a synergistic antitumor effect in HER2-postive gastric cancer cells. Anticancer Drugs 2014;25:315-22. [Crossref] [PubMed]
- Shirasaka T. Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol 2009;39:2-15. [Crossref] [PubMed]
- Wu FL, Lu DC, Ying YP, et al. A Meta-analysis Reveals S-1-based Chemotherapy Improves the Survival of Patients With Advanced Gastric Cancer. Medicine (Baltimore) 2015;94:e652. [Crossref] [PubMed]
- Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 2009;10:1063-9. [Crossref] [PubMed]
- Kataoka H, Mori Y, Shimura T, et al. A phase II prospective study of the trastuzumab combined with 5-weekly S-1 and CDDP therapy for HER2-positive advanced gastric cancer. Cancer Chemother Pharmacol 2016;77:957-62. [Crossref] [PubMed]
- Chua C, Tan IB, Yamada Y, et al. Phase II study of trastuzumab in combination with S-1 and cisplatin in the first-line treatment of human epidermal growth factor receptor HER2-positive advanced gastric cancer. Cancer Chemother Pharmacol 2015;76:397-408. [Crossref] [PubMed]
- Kurokawa Y, Sugimoto N, Miwa H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer 2014;110:1163-8. [Crossref] [PubMed]
- Hacioglu B, Akin S, Babacan T, et al. How long should we maintain anti-HER2 therapy for metastatic breast cancer patients with complete remission? Future Oncol 2015;11:2799-801. [Crossref] [PubMed]
- Ihnenfeld Arciénega I, Imesch P, Fink D, et al. Prolonged complete remission of metastatic HER2-positive breast cancer after continuous trastuzumab treatment: a case report and review of the literature. Target Oncol 2015;10:297-301. [Crossref] [PubMed]
- Palacio S, Loaiza-Bonilla A, Kittaneh M, et al. Successful use of Trastuzumab with anthracycline-based chemotherapy followed by trastuzumab maintenance in patients with advanced HER2-positive gastric cancer. Anticancer Res 2014;34:301-6. [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Colon Cancer Version 2. 2017. Available online: https://www.nccn.org/professionals/physician_gls/recently_updated.aspx
- Al-Shamsi HO, Fahmawi Y, Dahbour I, et al. Continuation of trastuzumab beyond disease progression in HER2-positive metastatic gastric cancer: the MD Anderson experience. J Gastrointest Oncol 2016;7:499-505. [Crossref] [PubMed]
- Palle J, Tougeron D, Pozet A, et al. Trastuzumab beyond progression in patients with HER2-positive advanced gastric adenocarcinoma: A multicenter AGEO study. J Clin Oncol 2017;35:abstr 94.
- Li Q, Jiang H, Li H, et al. Efficacy of trastuzumab beyond progression in HER2 positive advanced gastric cancer: a multicenter prospective observational cohort study. Oncotarget 2016;7:50656-65. [PubMed]
- Chien AJ, Cockerill A, Fancourt C, et al. A phase 1b study of the Akt-inhibitor MK-2206 in combination with weekly paclitaxel and trastuzumab in patients with advanced HER2-amplified solid tumor malignancies. Breast Cancer Res Treat 2016;155:521-30. [Crossref] [PubMed]
- Hudis C, Swanton C, Janjigian YY, et al. A phase 1 study evaluating the combination of an allosteric AKT inhibitor (MK-2206) and trastuzumab in patients with HER2-positive solid tumors. Breast Cancer Res 2013;15:R110. [Crossref] [PubMed]
- Nishikawa K, Takahashi T, Takaishi H, et al. Phase II study of the effectiveness and safety of trastuzumab and paclitaxel for taxane- and trastuzumab-naive patients with HER2-positive, previously treated, advanced, or recurrent gastric cancer (JFMC45-1102). Int J Cancer 2017;140:188-96. [Crossref] [PubMed]
- Barok M, Tanner M, Koninki K, et al. Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett 2011;306:171-9. [Crossref] [PubMed]
- Krop IE, Kim SB, Gonzalez-Martin A, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:689-99. [Crossref] [PubMed]
- Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017;18:640-53. [Crossref] [PubMed]
- Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513-8. [Crossref] [PubMed]
- Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306-14. [Crossref] [PubMed]
- Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78-86. [Crossref] [PubMed]
- Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31:4438-44. [Crossref] [PubMed]
- Tanabe K, Fujii M, Nishikawa K, et al. Phase II/III study of second-line chemotherapy comparing irinotecan-alone with S-1 plus irinotecan in advanced gastric cancer refractory to first-line treatment with S-1 (JACCRO GC-05). Ann Oncol 2015;26:1916-22. [Crossref] [PubMed]
- Kang YK, Ryu MH, Park SH, et al. Efficacy and safety findings from DREAM: A phase III study of DHP107 (oral paclitaxel) vs. IV paclitaxel in patients with gastric cancer after failure of first-line chemotherapy. J Clin Oncol 2016;34:abstr 4016.
- Shitara K, Takashima A, Fujitani K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:277-87. [Crossref] [PubMed]
- Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039-49. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Pavlakis N, Sjoquist KM, Martin AJ, et al. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J Clin Oncol 2016;34:2728-35. [Crossref] [PubMed]
- Dutton SJ, Ferry DR, Blazeby JM, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol 2014;15:894-904. [Crossref] [PubMed]
- Ohtsu A, Ajani JA, Bai YX, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 2013;31:3935-43. [Crossref] [PubMed]
- Bang YJ, Cutsem EV, Mansoor W, et al. A randomized, open-label phase II study of AZD4547 (AZD) versus Paclitaxel (P) in previously treated patients with advanced gastric cancer (AGC) with Fibroblast Growth Factor Receptor 2 (FGFR2) polysomy or gene amplification (amp): SHINE study. J Clin Oncol 2015;33:abstr 4014.
- Bang YJ, Boku N, Chin K, et al. Gastrointestinal tumours, non-colorectalOlaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy: Phase III GOLD study. Ann Oncol 2016;27:LBA25. [Crossref]
- Wainberg ZA, Anghel A, Desai AJ, et al. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res 2010;16:1509-19. [Crossref] [PubMed]
- Iqbal S, Goldman B, Fenoglio-Preiser CM, et al. Southwest Oncology Group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol 2011;22:2610-5. [Crossref] [PubMed]
- Oh DY, Bang YJ. Pertuzumab in gastrointestinal cancer. Expert Opin Biol Ther 2016;16:243-53. [Crossref] [PubMed]
- Yamashita-Kashima Y, Iijima S, Yorozu K, et al. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res 2011;17:5060-70. [Crossref] [PubMed]
- Jørgensen JT. Role of human epidermal growth factor receptor 2 in gastric cancer: biological and pharmacological aspects. World J Gastroenterol 2014;20:4526-35. [Crossref] [PubMed]
- Janjigian YY, Viola-Villegas N, Holland JP, et al. Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and 89Zr-trastuzumab PET. J Nucl Med 2013;54:936-43. [Crossref] [PubMed]
- Janjigian YY, Ku GY, Ilson DH, et al. A phase II study of afatinib in patients (pts) with metastatic human epidermal growth factor receptor (HER2)-positive trastuzumab refractory esophagogastric (EG) cancer. J Clin Oncol 2015;33:abstr 59.
- Nam HJ, Ching KA, Kan J, et al. Evaluation of the antitumor effects and mechanisms of PF00299804, a pan-HER inhibitor, alone or in combination with chemotherapy or targeted agents in gastric cancer. Mol Cancer Ther 2012;11:439-51. [Crossref] [PubMed]
- Oh DY, Lee KW, Cho JY, et al. Phase II trial of dacomitinib in patients with HER2-positive gastric cancer. Gastric Cancer 2016;19:1095-103. [Crossref] [PubMed]
- Nordstrom JL, Gorlatov S, Zhang W, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res 2011;13:R123. [Crossref] [PubMed]
- Bang YJ, Giaccone G, Im SA, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol 2017;28:855-61. [PubMed]
- McDonagh CF, Huhalov A, Harms BD, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther 2012;11:582-93. [Crossref] [PubMed]
- Doi T, Iwata H, Tsurutani J, et al. Single agent activity of DS-8201a, a HER2-targeting antibody-drug conjugate, in heavily pretreated HER2 expressing solid tumors. J Clin Oncol 2017;35:abstr 108.
- Bleiberg H, Conroy T, Paillot B, et al. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer 1997;33:1216-20. [Crossref] [PubMed]
- Mitry E, Taieb J, Artru P, et al. Combination of folinic acid, 5-fluorouracil bolus and infusion, and cisplatin (LV5FU2-P regimen) in patients with advanced gastric or gastroesophageal junction carcinoma. Ann Oncol 2004;15:765-9. [Crossref] [PubMed]
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-v49. [Crossref] [PubMed]
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991-7. [Crossref] [PubMed]
- Shah MA, Janjigian YY, Stoller R, et al. Randomized Multicenter Phase II Study of Modified Docetaxel, Cisplatin, and Fluorouracil (DCF) Versus DCF Plus Growth Factor Support in Patients With Metastatic Gastric Adenocarcinoma: A Study of the US Gastric Cancer Consortium. J Clin Oncol 2015;33:3874-9. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. J Clin Oncol 2017;35:abstr 4004.
- Ter Veer E, Haj Mohammad N, van Valkenhoef G, et al. Second- and third-line systemic therapy in patients with advanced esophagogastric cancer: a systematic review of the literature. Cancer Metastasis Rev 2016;35:439-56. [Crossref] [PubMed]
- Bando H, Doi T, Muro K, et al. A multicenter phase II study of TAS-102 monotherapy in patients with pre-treated advanced gastric cancer (EPOC1201). Eur J Cancer 2016;62:46-53. [Crossref] [PubMed]
- Shih CH, Ozawa S, Ando N, et al. Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res 2000;6:1161-8. [PubMed]
- Enzinger PC, McCleary NJ, Zheng H, et al. Multicenter double-blind randomized phase II: FOLFOX + ziv-aflibercept/placebo for patients (pts) with chemo-naive metastatic esophagogastric adenocarcinoma (MEGA). J Clin Oncol 2016;34:abstr 4.
- Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter Phase II trial. Ann Oncol 2016;27:2196-203. [Crossref] [PubMed]
- van Grieken NC, Aoyama T, Chambers PA, et al. KRAS and BRAF mutations are rare and related to DNA mismatch repair deficiency in gastric cancer from the East and the West: results from a large international multicentre study. Br J Cancer 2013;108:1495-501. [Crossref] [PubMed]
- Zheng H, Wang Y, Tang C, et al. TP53, PIK3CA, FBXW7 and KRAS Mutations in Esophageal Cancer Identified by Targeted Sequencing. Cancer Genomics Proteomics 2016;13:231-8. [PubMed]
- Suntharalingam M, Winter K, Ilson D, et al. Effect of the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation Therapy for Patients With Esophageal Cancer: The NRG Oncology RTOG 0436 Phase 3 Randomized Clinical Trial. JAMA Oncol 2017;3:1520-8. [Crossref] [PubMed]
- Du F, Zheng Z, Shi S, et al. S-1 and Cisplatin With or Without Nimotuzumab for Patients With Untreated Unresectable or Metastatic Gastric Cancer: A Randomized, Open-Label Phase 2 Trial. Medicine (Baltimore) 2015;94:e958. [Crossref] [PubMed]
- Satoh T, Lee KH, Rha SY, et al. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric Cancer 2015;18:824-32. [Crossref] [PubMed]
- Dragovich T, McCoy S, Fenoglio-Preiser CM, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol 2006;24:4922-7. [Crossref] [PubMed]
- Wainberg ZA, Lin LS, DiCarlo B, et al. Phase II trial of modified FOLFOX6 and erlotinib in patients with metastatic or advanced adenocarcinoma of the oesophagus and gastro-oesophageal junction. Br J Cancer 2011;105:760-5. [Crossref] [PubMed]
- Pyo JS, Kang G, Cho H. Clinicopathological Significance and Diagnostic Accuracy of c-MET Expression by Immunohistochemistry in Gastric Cancer: A Meta-Analysis. J Gastric Cancer 2016;16:141-51. [Crossref] [PubMed]
- Singh P, Toom S, Huang Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol 2017;10:105. [Crossref] [PubMed]
- Cafferkey C, Chau I. Novel STAT 3 inhibitors for treating gastric cancer. Expert Opin Investig Drugs 2016;25:1023-31. [Crossref] [PubMed]
- Becerra C, Stephenson J, Jonker DJ, et al. Phase Ib/II study of cancer stem cell (CSC) inhibitor BBI608 combined with paclitaxel in advanced gastric and gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol 2015;33:abstr 4069.
- Shah MA, Starodub A, Berlin J, et al. Updated results of a phase 1 study combining the matrix metalloproteinase 9 inhibitor GS-5745 and mFOLFOX6 in patients with advanced gastric/gastroesophageal junction cancer. J Clin Oncol 2017.35. abstract 108.
- Luke JJ, Flaherty KT, Ribas A, et al. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14:463-82. [Crossref] [PubMed]
- Giroux Leprieur E, Dumenil C, Julie C, et al. Immunotherapy revolutionises non-small-cell lung cancer therapy: Results, perspectives and new challenges. Eur J Cancer 2017;78:16-23. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Lote H, Cafferkey C, Chau I. PD-1 and PD-L1 blockade in gastrointestinal malignancies. Cancer Treat Rev 2015;41:893-903. [Crossref] [PubMed]
- Xu F, Feng G, Zhao H, et al. Clinicopathologic Significance and Prognostic Value of B7 Homolog 1 in Gastric Cancer: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015;94:e1911. [Crossref] [PubMed]
- Janjigian YY, Ott PA, Calvo E, et al. Nivolumab ± ipilimumab in pts with advanced (adv)/metastatic chemotherapy-refractory (CTx-R) gastric (G), esophageal (E), or gastroesophageal junction (GEJ) cancer: CheckMate 032 study. J Clin Oncol 2017;35:abstr 4014.
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RWJ, et al. KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. J Clin Oncol 2017;35:abstr 4003.
- Bang YJ, Muro K, Fuchs CS, et al. KEYNOTE-059 cohort 2: Safety and efficacy of pembrolizumab (pembro) plus 5-fluorouracil (5-FU) and cisplatin for first-line (1L) treatment of advanced gastric cancer. J Clin Oncol 2017;35:abstr 4012.
- Chung HC, Arkenau HT, Wyrwicz L, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced gastric or gastroesophageal junction cancer from JAVELIN solid tumor phase Ib trial: Analysis of safety and clinical activity. J Clin Oncol 2016;34:abstr 4009.
- Dempke WCM, Fenchel K, Uciechowski P, et al. Second- and third-generation drugs for immuno-oncology treatment-The more the better? Eur J Cancer 2017;74:55-72. [Crossref] [PubMed]
- Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nat Rev Immunol 2017;17:703-17. [Crossref] [PubMed]
- Böger C, Behrens HM, Kruger S, et al. The novel negative checkpoint regulator VISTA is expressed in gastric carcinoma and associated with PD-L1/PD-1: A future perspective for a combined gastric cancer therapy? Oncoimmunology 2017;6:e1293215. [Crossref] [PubMed]
- Zhang R, Li H, Yu J, et al. Immunoactivative role of indoleamine 2,3dioxygenase in gastric cancer cells in vitro. Mol Med Rep 2011;4:169-73. [PubMed]
- Liu H, Shen Z, Wang Z, et al. Increased expression of IDO associates with poor postoperative clinical outcome of patients with gastric adenocarcinoma. Sci Rep 2016;6:21319. [Crossref] [PubMed]
- Soliman HH, Minton SE, Han HS, et al. A phase I study of indoximod in patients with advanced malignancies. Oncotarget 2016;7:22928-38. [Crossref] [PubMed]
- Soliman HH, Jackson E, Neuger T, et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget 2014;5:8136-46. [Crossref] [PubMed]
- Burris HA, Gordon MS, Hellmann MD, et al. A phase Ib dose escalation study of combined inhibition of IDO1 (GDC-0919) and PD-L1 (atezolizumab) in patients (pts) with locally advanced or metastatic solid tumors. J Clin Oncol 2017;35:abstr 105.
- Placke T, Kopp HG, Salih HR. Glucocorticoid-induced TNFR-related (GITR) protein and its ligand in antitumor immunity: functional role and therapeutic modulation. Clin Dev Immunol 2010;2010:239083.
- Le DT, Durham JN, Smith KN, et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Analysis Working Group: Asan University; BC Cancer Agency; et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169-75. [Crossref] [PubMed]
- Nonagase Y, Yonesaka K, Kawakami H, et al. Heregulin-expressing HER2-positive breast and gastric cancer exhibited heterogeneous susceptibility to the anti-HER2 agents lapatinib, trastuzumab and T-DM1. Oncotarget 2016;7:84860-71. [Crossref] [PubMed]
- Wang H, Wang W, Xu Y, et al. Aberrant intracellular metabolism of T-DM1 confers T-DM1 resistance in HER2-positive gastric cancer cells. Cancer Sci 2017;108:1458-68. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Charoentong P, Finotello F, Angelova M, et al. Pan-cancer Immunogenomic Analyses Reveal Genotype-Immunophenotype Relationships and Predictors of Response to Checkpoint Blockade. Cell Rep 2017;18:248-62. [Crossref] [PubMed]
- André F, O'Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2014;15:580-91. [Crossref] [PubMed]
- Lopez-Albaitero A, Xu H, Guo H, et al. Overcoming resistance to HER2-targeted therapy with a novel HER2/CD3 bispecific antibody. Oncoimmunology 2017;6:e1267891. [Crossref] [PubMed]
- Chau I, Bendell JC, Calvo E, et al. Interim safety and clinical activity in patients (pts) with advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma from a multicohort phase 1 study of ramucirumab (R) plus pembrolizumab (P). J Clin Oncol 2017;35:abstr 102.


