Cardiovascular outcome studies with glucagon-like peptide 1 receptor agonists—what will REWIND add?
Type 2 diabetes mellitus (T2DM) is a complex metabolic disease with hyperglycemia and an associated high risk of cardiovascular disease (CVD), including macrovascular and microvascular complications. In addition, CVD mortality rates appear two fold higher among individuals with diabetes mellitus (DM) when compared to individuals without DM (1,2).
Moreover, along with myocardial infarction (MI) and stroke, persons with diabetes have been shown to have an increased risk for hospitalization from heart failure (HF). In the July 2017 edition of Diabetes Obesity and Metabolism, Gerstein et al. (3) delineate the design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) study. The REWIND trial aims to determine whether the addition of a once weekly injection of a glucagon-like peptide-1 receptor agonist (GLP-1 RA), dulaglutide to other antidiabetic medications in middle-aged and older individuals with T2DM safely reduces the incidence of cardiovascular (CV) outcomes. This editorial is written to elucidate potential benefits and shortcomings of the REWIND design, baseline population and to add to the understanding of the potential of GLP-1 RAs in reducing CVD in presence of DM. At the present time, several GLP-1 RAs have been developed and used in clinical care and/or research, including liraglutide, lixisenatide, exenatide, dulaglutide, semaglutide, albiglutide and, more recently implanted subcutaneous exenatide pump.
Improved glycemic control has been demonstrated to decrease microvascular outcomes (4), yet a conclusive beneficial effect on macrovascular outcomes is elusive, except for recent data with liraglutide and empagliflozin, and to a lesser extent semaglutide. Nevertheless, data collected over the last 10 years suggest that the risk of macrovascular complications increases with the severity of abnormalities of blood glucose. Therefore, it can be hypothesized that, similar to outcome studies treating hypertension and hypercholesterolemia, an approach aimed at reducing the hyperglycemic burden should result in a clear-cut reduction of CV events in the population with DM. Unfortunately, the relation between glucose-lowering pharmacotherapy and CVD is much more complex than is the case with modification of other CV risk factors. Various studies suggest that, despite being effective in lowering the glucose, some hypoglycemic agents may potentially increase CV events (5,6). A meta-analysis by Nissen and Wolski concluded that rosiglitazone was associated with a significant increase in the risk of MI (5). This evidence led to the withdrawal of rosiglitazone in Europe and restricted its use in the United States (US).
Furthermore, these findings prompted the reevaluation of the approval processes for all new antidiabetic medications. Since 2008, US Food and Drug Administration (FDA) has requested rigorous CV outcome data from randomized, controlled trials in order to grant and sustain approvals of new drugs for the treatment of DM (7). Therefore, although the primary CV outcome for the REWIND trial is the first occurrence of the composite of either CV death or non-fatal MI or nonfatal stroke, the trial is also explicitly assessing potential side effects of dulaglutide and its effect on a large variety of clinically important outcomes including all-cause mortality, renal disease, hospitalizations for HF or angina, cancer (including thyroid cancer), and pancreatitis.
Multiple classes of anti-diabetic agents have been studied for CV events, with mixed results. Significantly, insulin and sulfonylureas (SU) in United Kingdom Prospective Diabetes Study (UKPDS) 33 Origin trial had neutral outcomes (4). Several decades prior to the present time, there was concern about the safety profile of the SU, despite apparent reduction of microvascular complications. So far, in trials that have evaluated CV outcomes associated with newer glucose-lowering agents, no significant effect on major adverse CV events (MACE) has been shown other than within the approved three dipeptidyl peptidase 4 (DPP-4) inhibitors (saxagliptin, alogliptin, and sitagliptin) (8-10), an apparent increased new-onset HF with saxagliptin and alogliptin leading to warning in US drug labelling.
In addition, one GLP-1 RA (lixisenatide) (11) has shown neutral CV outcomes, but a lower risk of MACE was shown with two other GLP-1 RAs (liraglutide and semaglutide) (12,13) and empagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor (14). Another agent from this class, canagliflozin, had a lower risk of CV events than those who received placebo but a greater risk of amputation, primarily at the level of the toe or metatarsal (15).
In consideration of the above, the REWIND trial design, baseline characteristics and eventual outcomes are of great interest to researchers and practicing clinicians. GLP-1 RAs have the ability to mimic endogenous GLP-1, resulting in a glucose-dependent increase in insulin secretion and an inhibition of glucagon secretion. They are well tolerated while inducing modest, long-lasting reductions of hemoglobin (Hb)A1c, with the most common side effect being transient nausea. The GLP-1 receptor is expressed in the vascular endothelium and cardio-myocytes, suggesting that they may have an impact on CVD, improving insulin sensitivity, left ventricular remodeling, and cardiac contractility in models of chronic HF and MI (16,17). GLP-1 RAs have also demonstrated a favorable impact on several CV risk factors such as body weight, blood pressure, endothelial function, and low density lipoprotein in patient with and without diabetes (16).
The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial investigated the effects of lixisenatide versus placebo in 6,068 patients with diabetes. It concluded non-inferiority of lixisenatide to placebo. However, the study did not show superiority as far as CV outcome is concerned (11). Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes (LEADER) trial had enrolled 9,340 patients with a median follow up period of 3.8 years. Patient characteristics included mean HbA1c of 8.7%, mean BMI of 33 kg/m2 and 81% of the participants had prior documented CVD. This was a high-risk population and expectedly demonstrated lower rates of first occurrence of death from CV causes, nonfatal MI, or nonfatal stroke among patients with T2DM, with liraglutide than with placebo (12). Based on these positive findings, FDA has approved liraglutide injection to reduce the risk of MACE in adults with T2DM and established CV disease.
In addition, a Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) among 3,297 patients with T2DM at high CV risk, the rate of first occurrence of death from CV causes, nonfatal MI, or nonfatal stroke was significantly lower in those receiving semaglutide than in those receiving placebo, which confirmed non inferiority. This agent is not approved for use in the US (13).
The recently published Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes (EXSCEL) trial demonstrated non-inferiority of exenatide over placebo with respect to safety (P<0.001 for non-inferiority) but was not superior to placebo with respect to efficacy (P=0.06 for superiority) (18). EXSCEL had a large sample size of 14,752 patients who were followed for median period of 3.2 years, with a mean HbA1c of 8.2%, and 70% of the participants had prior documented CVD. Furthermore, lipid-lowering medications and SGLT-2 inhibitors were used with increased frequency in the placebo arm verses the treatment arm, potentially a major reason for the neutral results regarding efficacy.
The agent in REWIND, dulaglutide, a synthetic analogue of human GLP-1, given once weekly has shown potential beneficial effects on blood pressure, despite a small increase in heart rate (19). In 755 persons, dulaglutide 1.5 mg was associated with reduction in 24-hour SBP 2.8 mmHg (P≤0.001). The mechanism for the response observed in this effect remains unclarified. Encouraging data were also reported from a meta-analysis of prior retrospective studies suggested CV safety with dulaglutide. This meta-analysis by Ferdinand et al. 2016 analyzed 6,010 randomized patients, 3,885 to dulaglutide and 2,125 to comparator therapy, with median follow up of 1 year. Primary outcome of all-cause mortality analysis demonstrated hazard ratio (HR) <1. Therefore, while awaiting final results of REWIND, thus far dulaglutide has not been shown to increase the risk of major CV events in T2DM patients (20).
The sample size in REWIND is the largest second only to the recently published EXSCEL trial among studies that evaluated antidiabetic medication in CVD outcome. The size of the study is robust with a recruitment of 9,901 participants (mean age 66) occurred in 370 sites located in 24 countries. Fortunately, admirable 46% of the subjects were women. Sample size calculations were based on a 3-year recruitment period, an anticipated primary outcome event rate of 2% per year in the control group, annual dropout rate of 0.15%, and a 2-sided type 1 error of 5%. These assumptions indicated that recruitment of 9,600 patients would result in a total of 1,200 participants with at least one primary CV outcome over a maximum follow-up period of 8 years, and will provide 90% power to detect a HR of 0.82 for CV events when compared with other recent GLP-1 studies. This high number of outcome events also ensures that there is a possibility of a narrow confidence interval (CI) around the estimated end point parameters.
Additionally, a component of the REWIND design is that it appears to reflect a population which, although at increased risk, is more consistent with the majority of patients seen in usual clinical practice as compared to other GLP-1RA studies which included a higher-risk population. The mean REWIND baseline HbA1c was 7.3% and 31% had prior CVD, reflective of most middle-aged and older persons with T2DM. In the LEADER and EXSCEL trials, 81% and 73% respectively patients had prior CVD at the time of enrollment, which is higher as compared to 31% in the REWIND. Furthermore, the mean HbA1c was 7.3, much lower than the other GLP1-RA studies, LEADER (HbA1c 8.7%) and SUSTAIN 6 (HbA1c of 8.7%) and EXSCEL (HbA1c 8.0%) (Table 1).
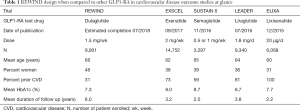
Full table
The pragmatic design of the trial included integration with usual care and wide-ranging eligibility criteria. All efficacy and safety analyses will be conducted using an intention-to-treat approach that includes all randomized participants regardless of adherence.
The composition of the population enrolled in a trial should help FDA reviewers, clinicians, or policy makers to have confidence that the trial results will apply to future practice. Historically, the elderly, women, and racial/ethnic minorities have been underrepresented in trials. A substantial body of literature has documented this under-representation in recent years, particularly for women in some CV trials and general inclusion of black/African-American and minority participants in clinical trials. In response to these concerns, Congress included Section 907 in the FDA Safety and Innovation Act of 2012, giving FDA direction to evaluate this issue and take action (21). Of note, there is 46% female representation in the REWIND trial which is higher than other recent CV trials.
Additionally, among the GLP-1RA studies, REWIND has the longest follow up period. The EXSCEL trial had a median 3.2 years of follow up while LEADER trial had median follow up of 3.8 years. REWIND provides a median follow up time of 8 years, which has been calculated to be adequate to register the required events in a population with a relatively lower baseline CVD.
While REWIND’s international scope is attractive, there is also the consideration that clinicians in North America will have some concern about the applicability of the 20.9% patients who are not seen in the US and Canada. For instance, a subgroup analysis of Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial suggested that patients in Russia and Georgia were not taking their study agent, adding to the documented regional variation in outcomes (22). Indeed, the large proportion of study patients outside of North America suggest that a REWIND sub-group analysis should be done to reveal potential lapses in clinical research appropriateness if any.
The lack of CV efficacy in the EXSCEL trial may be a cause of concern. Factors that lead to non-superior efficacy result of EXSCEL was probably due to short median follow-up time, short duration of exposure to the trial regimen, and the high rate of discontinuation of the trial regimen. The disproportionate use in the placebo group of anti-diabetic therapies known to reduce CV risk, such as SGLT-2 inhibitors and GLP-1 receptor agonists, may have preferentially resulted in lower event rates in the placebo group (18). In light of the above data, REWIND trial should account for these factors while reporting the results.
Lastly, minority groups in the US remain disproportionately affected by DM. For instance, the prevalence of DM (diagnosed DM or HbA1c ≥6.5%) in non-Hispanic blacks is almost twice as high as in whites (15.4% versus 8.6%) (2). As per the results from the EXSCEL trial there was clear trend of increased benefit to the black population HR (CI) 0.67 (0.45–0.99). Though the black representation in the sample size was much smaller, the benefits of exenatide in reducing CVD outcomes was greater than the white population and in this case reached statistical significance as well (18). This calls for a greater enrollment and representation of this population which may benefit from an antidiabetic agent with positive blood pressure lowering effect in the future studies. Minority underrepresentation in CV outcome trials for T2DM remains a concern which REWIND may not fully address. As treatment choices for type 2 diabetes evolve from a one size-fit-all approach into a model based on patient-centered precision medicine, there is a need for deeper understanding of the clinically meaningful pharmaco-genomic and pharmacokinetic differences between individuals receiving such therapies to determine if there are variations in their potential effect, including differences in CV outcomes for different racial and ethnic groups.
In conclusion, the advent of the novel anti-diabetic medications provides new horizons in the field of diabetes and CVD. The protocol for REWIND is well designed, with a large sample size, a long follow up period and adequate outcome event to ensure a narrow CI. At this point, the most widely reported outcomes study with a GLP-1 RA is the LEADER trial which studied a high-risk population and had a higher mean HbA1c, prior CVD when compared to REWIND population. Therefore, being a relatively lower risk population portends that the final reported results of REWIND may be less robust, although the applicability to general practice will be more acceptable. The final result of REWIND will provide a complete evaluation of the clinical effects and safety of dulaglutide and will facilitate the ability of physicians to practice evidence-based medicine for patients with T2DM and increased CVD risk.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. KC Ferdinand is a consultant for Amgen, Sanofi, Boehringer-Ingelheim, Quantum Genomics, and Novatis. Dr. I Mahata has no conflicts of interest to declare.
References
- Tancredi M, Rosengren A, Svensson AM, et al. Excess Mortality among Persons with Type 2 Diabetes. N Engl J Med 2015;373:1720-32. [Crossref] [PubMed]
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146-e603. [Crossref] [PubMed]
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837-53. [Crossref] [PubMed]
- Nissen SE, Wolski K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. N Engl J Med 2007;356:2457-71. [Crossref] [PubMed]
- Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 2007;370:1129-36. [Crossref] [PubMed]
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry. Diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention. Clinical/Medical 2008.1-30.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N Engl J Med 2013;369:1317-26. [Crossref] [PubMed]
- White WB, Cannon CP, Heller SR, et al. Alogliptin after Acute Coronary Syndrome in Patients with Type 2 Diabetes. N Engl J Med 2013;369:1327-35. [Crossref] [PubMed]
- Green JB, Bethel MA, Armstrong PW, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2015;373:232-42. [Crossref] [PubMed]
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med 2015;373:2247-57. [Crossref] [PubMed]
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2016;375:311-22. [Crossref] [PubMed]
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2016;375:1834-44. [Crossref] [PubMed]
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015;373:2117-28. [Crossref] [PubMed]
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017;377:644-57. [Crossref] [PubMed]
- Paneni F, Lüscher TF. Cardiovascular Protection in the Treatment of Type 2 Diabetes: A Review of Clinical Trial Results Across Drug Classes. Am J Med 2017;130:S18-S29. [Crossref] [PubMed]
- Sokos GG, Nikolaidis LA, Mankad S, et al. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail 2006;12:694-9. [Crossref] [PubMed]
- Holman RR, Bethel MA, Mentz RJ, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2017;377:1228-39. [Crossref] [PubMed]
- Ferdinand KC, White WB, Calhoun DA, et al. Effects of the Once-Weekly Glucagon-Like Peptide-1 Receptor Agonist Dulaglutide on Ambulatory Blood Pressure and Heart Rate in Patients With Type 2 Diabetes Mellitus. Hypertension 2014;64:731-7. [Crossref] [PubMed]
- Ferdinand KC, Botros FT, Atisso CM, et al. Cardiovascular safety for once-weekly dulaglutide in type 2 diabetes: a pre-specified meta-analysis of prospectively adjudicated cardiovascular events. Cardiovasc Diabetol 2016;15:38. [Crossref] [PubMed]
- United States Congress. Food and Drug Administration Safety and Innovation Act. 2012;S3187:1-140.
- de Denus S, O’Meara E, Desai AS, et al. Spironolactone Metabolites in TOPCAT — New Insights into Regional Variation. N Engl J Med 2017;376:1690-2. [Crossref] [PubMed]


