Predictors of response and resistance to checkpoint inhibitors in solid tumors
Checkpoint inhibitors (blocking antibodies to PD-1, PD-L1, CTLA-4) have proven effective against several tumor types. Unfortunately, only a minority of tumors within each subtype responds, and checkpoint inhibitors can cause significant toxicity. There is a flurry of activity around determining patient and tumor factors of response and resistance to this class of medication. To date, most of the data surrounds mutational burden and amount of PD-L1 staining in pre-treatment samples.
Roh et al. examined clinical and correlative characteristics of advanced melanoma patients as their melanoma progressed through treatments with CTLA-4 inhibitors and PD-1 inhibitors. Of an initial cohort of 56 patients, 54 were treated with CTLA-4 inhibitor, and 7 of them responded (1). The non-responders were then treated with the PD-1 inhibitor, and 14 (28%) responded (1). Using serial biopsies, the authors identified several tumor factors that favor response and resistance (1). Of note, their definition of response is either radiographic partial or complete remission or stable disease lasting at least 6 months (1).
The authors undertook an impressive level of investigation, building upon their previous report of deep immune profiling. In their sample, they did not find a correlation between number of mutations, tumor heterogeneity or T cell receptor clonality with response or resistance. However, they did find that a high number of copy losses was associated with lack of response to checkpoint inhibition. Specifically, they found that patients who progressed on both CTLA-4 and PD-1 blockade were more likely to have higher numbers of copy loss than those who responded to CTLA-4 blockade. They did not see an association between copy number loss and mutational burden. In examining the types of genes for which there were copy number alterations, they observed that those subjects who did not respond to either agent tended to have recurrent copy number loss in tumor suppressor genes on chromosomes 6q, 10q, and 11q23.3 (1).
To confirm that copy number loss is associated with lack of response to checkpoint inhibitions, they used whole exome sequencing from a separate cohort of 110 melanoma patients and RNA sequencing from data from 42 additional melanoma patients. For this analysis, copy number loss was not associated with resistance, but lower number of copy number loss was associated with benefit. While tumor suppressor genes continued to be a factor, the specific mutations were not overlapping. Using a previously described association between loss of chromosome 10 and resistance to CTLA-4 inhibitors, the authors found that loss of copy number on chromosome 10 was also associated with lack of response to CTLA-4, perhaps because PTEN resides on that chromosome, and PTEN loss is a putative driver of resistance.
This analysis is an important addition to the body of knowledge surrounding response and resistance in checkpoint inhibitors. Other studies of clinical specimens have shown that there are host and tumor factors that are linked to response and resistance (Table 1). Some of these factors can be easily examined on routine laboratory tests, whereas others require tissue biopsy. With the exception of PD-L1 expression and microsatellite instability, both of which are most reliably gauged using a tumor biopsy, none of these is in routine clinical use.
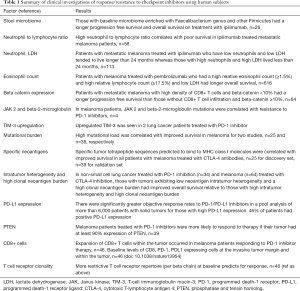
Full table
Checkpoint inhibitors are one mechanism by which to overcome tumor-associated immune suppression. However, the upregulation of these negative regulators on T cells is not the only mechanism by which the tumor evades an active immune response. In fact, tumors utilize multiple, often redundant, “layers” of immune suppression. Blood vessels within the tumor but not adjacent normal tissue express the death mediator Fas Ligand (FasL) as a mechanism by which to kill tumor infiltrating Fas+CD8 T cells (2). Interestingly, CD4+Foxp3+ suppressive cells express lower levels of Fas thereby protecting them from FasL mediated apoptosis (2). Tumor cells also develop mechanisms to resist immune cell killing. Some of the mechanisms described include the upregulation of IAPs (inhibitors of apoptosis) (3), increased expression of pro-survival genes such as BCL2/BCL-XL (3), downregulation of MHC I on the tumor cell surface (4) and upregulation of T cell inhibitory molecules such as PD-L1. In addition, because tumor cells undergo high rates of proliferation, they also have demanding metabolic needs. This rapid consumption of extracellular nutrients leads to a hostile, hypoxic, microenvironment for immune cells (5) and starves cytotoxic T cells of the necessary metabolic substrates needed for optimal effector function (6). Aside from tumor cells, the tumor microenvironment is a highly inflammatory environment rich in stromal cells and leukocytes that secrete inhibitory molecules such as TGF-β (7), IDO (8), IL-10 (9), and arginase (10). Therefore, effective immunotherapies must overcome multiple layers of immune cell suppression within the tumor microenvironment. While checkpoint blockade targets one of these layers, combination therapies that target multiple layers may find synergy in the clinic.
Host factors of response and resistance
Studies of host factors have identified composition of the gut microbiome through characterization of stool specimens at baseline is associated with response. In a study of 26 patients receiving ipilimumab for metastatic melanoma, fecal microbiota was assessed using 16S ribosomal RNA sequencing before and after ipilimumab infusion. Researchers found that patients with progression free survival of more than 6 months had a significant presence of Faecalibacterium genus and other Firmicutes bacteria in their microbiota compared to non-responders in whom Bacteroides was predominant. Autoimmune colitis was found more in patients with a high proportion of Firmicutes in their microbiota, while no colitis occurred in Bacteroides predominant microbiota patients. It is intriguing that the gut microbiota could have a significant effect on checkpoint inhibitor therapeutic efficacy and adverse effects. Some have proposed manipulation of gut microbiota prior to treatment administration to maximize therapeutic efficacy and minimize adverse effects. Fecal transplant is a recognized treatment for antibiotic refractory Clostridium difficile infections and may be utilized for patients undergoing checkpoint inhibitor therapy (11).
Aging results in thymic involution suggesting that aging results in a systemic reduction in number of T-cells and worse antitumor response. One study testing the effect on age on ipilimumab efficacy found that age did not significantly affect response to ipilimumab. The study was conducted on 188 patients over the age of 70 with unresectable advanced (stage III, stage IV) melanoma and found an immune related disease control rate (immune related complete response/partial response/stable disease) of over 3 months in 38% of elderly patients compared to an immune related disease control of 33% in 645 patients less than 70. The differences between age groups in median progression free survival and overall survival were not statistically significant (over 70, n=193; less than 70, n=662). The results of this study suggest that age is inconsequential factor on checkpoint inhibitor therapeutic efficacy (12).
Certain laboratory biomarkers can be predictive of increased response to checkpoint inhibitor therapy. One study conducted on 58 melanoma patients treated with ipilimumab found that a neutrophil to lymphocyte ratio greater than or equal to four before treatment was correlated with worse overall survival. Another study also examining biomarkers for survival for 113 metastatic melanoma patients treated with ipilimumab found that low serum LDH and neutrophils were significantly correlated with patients who lived more than 2 years while high serum LDH and neutrophils were significantly correlated with patients who lived less than 2 years (13). Another study looked for biomarkers associated with overall survival in 616 melanoma patients treated with pembrolizumab and found that a high relative eosinophil count (≥1.5%), high relative lymphocyte count (≥17.5%), and low LDH were significantly correlated with better overall survival (14).
Another study tested the effect of ipilimumab on median overall survival in 187 HLA-A*0201-positive patients and 266 HLA-A*0201-negative patients (15). They found that median overall survival was similar in both patients, suggesting that the mechanism of ipilimumab is not dependent on HLA haplotype.
Tumor factors of response and resistance
Activation of the WNT pathway may be predictive of resistance to checkpoint inhibitor therapy due to downregulation of CD8+ T-cell infiltrate in the tumor microenvironment. One study testing the effect of baseline beta-catenin expression and prognosis of 64 metastatic melanoma patients found that greater beta-catenin expression correlated with decreased CD8+ T-cell infiltrate in the tumor microenvironment and vice versa. The results of this study suggest that activation of the Wnt pathway could be assessed before checkpoint inhibitor administration because adequate CD8+ T-cell presence is necessary in the tumor microenvironment for therapeutic efficacy (16).
JAK-2 and beta microglobulin mutations may be predictive of resistance to pembrolizumab, while mismatch repair deficiency mutations can be predictive of response to pembrolizumab. However, HLA status does not affect response to ipilimumab. Biopsy samples were analyzed from four relapsing patients with metastatic melanoma who progressed on pembrolizumab. Two of four patients had mutations in the JAK kinase while a third patient had a mutation in the beta-2-microglobulin antigen presenting protein. A cell line from a patient with a JAK2 mutation found lack of PD-L1 upregulation in response to stimulation with interferon gamma. Beta-2-microglobulin is part of the MHC I complex and mutations in beta-2-microglobulin resulted in loss of outer membrane localization of the MHC I complex which would result in subsequent immune evasion (17).
Another study compared the response to pembrolizumab in 32 patients with progressive metastatic colon cancer with or without mismatch repair deficiency (mismatch repair deficient, n=11; mismatch repair proficient, n=21) and 9 patients with mismatch repair tumors that were not colorectal. Patient with mismatch repair defects in their tumors had a significantly greater immune related objective response rate and progression free survival rate on pembrolizumab than patients without mismatch repair defects (18). The results suggest that mismatch repair defects are predictive of therapeutic efficacy in response to pembrolizumab.
Alternative immune checkpoint inhibitors in tumors can be predictive of resistance to checkpoint inhibitor therapy. One study assessed two lung patients who had progressed on pembrolizumab and compared their tumor antigens to five lung patients who had not been treated with immunotherapy. The two lung cancer patients treated with pembrolizumab had significant TIM-3 upregulation on the T-cells compared to patients who had not been treated with pembrolizumab (19). Perhaps pembrolizumab should be combined with an antibody to TIM-3 to maximize patients’ overall survival.
Higher mutational load in a tumor improves overall survival and could be predictive of response to checkpoint inhibitor therapy. Number of intratumoral mutations can influence overall survival. A study performed on 25 patients treated with ipilimumab aimed to elucidate whether the certain aspects of the tumor genome resulted in improved response to anti-CTLA-4 therapy. Whole exome sequencing compared DNA from tumors to normal blood from the same patient. Increased number of mutations was significantly correlated with improved survival and improved response to anti-CTLA-4 therapy; however, there were tumors with a large number of mutations that did not respond to therapy (20). Perhaps treatments prior to checkpoint inhibitor therapy should attempt to increase the number of mutations in tumors since a higher mutational load improves overall survival. In 2017, the Food and Drug Administration approved the use of pembrolizumab in any cancer that exhibits microsatellite instability (21).
The tumor landscape can be predictive of response to checkpoint inhibitor therapy. A study of 25 patients treated with ipilimumab also identified a highly significant association with peptide signatures of tumors and benefit from CTLA-4 blockade. The researchers searched for conserved stretches of amino acids predicted to bind to MHC class I molecules amongst multiple tumors. They identified many tetrapeptide sequences that were highly correlated with strong response to CTLA-4 blockade and increased overall survival. High mutational load likely increased the probability of formation of the tetrapeptide sequences (20).
Another study investigating the relationship between tumor landscape and response to checkpoint inhibitor therapy was conducted on 34 patients with non-small cell lung cancer treated with pembrolizumab. Researchers found that tumors that responded to pembrolizumab had significantly lower neoantigen intratumor heterogeneity than tumors with no clinical benefit. Overall, tumors with low neoantigen intratumor heterogeneity were associated with better overall survival (22).
A systematic review investigating the utility of using PD-L1 expression in solid tumors as a predictive biomarker of response to PD-1/PD-L1 inhibitors, compiled data from 41 clinical trials with 6,664 patients with solid tumors with a focus on objective response rates. The odds of patients responding to PD-1/PD-L1 inhibitor therapy with PD-L1 positive tumors were significantly higher than patients whose tumors were PD-L1 negative (23).
One study showed that PTEN loss correlated with poor response to PD-1 inhibitor treatment in 39 melanoma patients. Tumors with less than 10% of cells expressing PTEN via immunohistochemistry were classified as PTEN absent. Patients with PTEN present in their tumors had significantly greater tumor reduction than patients with PTEN absent (24).
Another study trying to elucidate patterns predictive for PD-1 inhibitor response in 46 patients with metastatic melanoma, found that tumors that responded to therapy had significantly more CD8 T-cells and PD-1 and PD-L1 expressing cells within it and at the invasive tumor margin as well as a more restrictive T cell receptor beta chain repertoire (25).
These studies suggest that the tumor landscape of the patient should be analyzed before administration of checkpoint inhibitor therapy for predictive peptide signatures, ligands, and low neoantigen intratumor heterogeneity.
We need reliable biomarkers to identify patients who are most likely (or even least likely) to respond to checkpoint inhibitors. Preferably these markers would not require biopsy, as some tumors cannot safely be biopsied. It is very likely that a single marker is insufficient and that an algorithm, or molecular signature, is needed. To definitively answer this question, we need more collaboration to increase the power of our analyses. Roh and colleagues are getting us one step closer.
Acknowledgements
None.
Footnote
Conflicts of Interest: JN Graff has received royalties from Oncoresponse, travel from Merck, consultation fees from Bayer and Dendreon. The other authors have no conflicts of interest to declare.
References
- Roh W, Chen PL, Reuben A, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med 2017;9:eaah3560. [Crossref] [PubMed]
- Motz GT, Santoro SP, Wang LP, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med 2014;20:607-15. [Crossref] [PubMed]
- Andersen MH, Becker JC, Straten Pt. Regulators of apoptosis: suitable targets for immune therapy of cancer. Nat Rev Drug Discov 2005;4:399-409. [Crossref] [PubMed]
- Ward PL, Koeppen HK, Hurteau T, et al. Major histocompatibility complex class I and unique antigen expression by murine tumors that escaped from CD8+ T-cell-dependent surveillance. Cancer Res 1990;50:3851-8. [PubMed]
- Barsoum IB, Smallwood CA, Siemens DR, et al. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 2014;74:665-74. [Crossref] [PubMed]
- Scharping NE, Menk AV, Moreci RS, et al. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity 2016;45:701-3. [Crossref] [PubMed]
- Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med 2001;7:1118-22. [Crossref] [PubMed]
- Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol 2003;24:242-8. [Crossref] [PubMed]
- Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942-9. [Crossref] [PubMed]
- Rodriguez PC, Quiceno DG, Zabaleta J, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res 2004;64:5839-49. [Crossref] [PubMed]
- Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017;28:1368-79. [Crossref] [PubMed]
- Chiarion Sileni V, Pigozzo J, Ascierto PA, et al. Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centres through the expanded access programme. J Exp Clin Cancer Res 2014;33:30. [Crossref] [PubMed]
- Valpione S, Martinoli C, Fava P, et al. Personalised medicine: Development and external validation of a prognostic model for metastatic melanoma patients treated with ipilimumab. Eur J Cancer 2015;51:2086-94. [Crossref] [PubMed]
- Weide B, Martens A, Hassel JC, et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin Cancer Res 2016;22:5487-96. [Crossref] [PubMed]
- Wolchok JD, Weber JS, Hamid O, et al. Ipilimumab efficacy and safety in patients with advanced melanoma: a retrospective analysis of HLA subtype from four trials. Cancer Immun 2010;10:9. [PubMed]
- Massi D, Romano E, Rulli E, et al. Baseline β-catenin, programmed death-ligand 1 expression and tumour-infiltrating lymphocytes predict response and poor prognosis in BRAF inhibitor-treated melanoma patients. Eur J Cancer 2017;78:70-81. [Crossref] [PubMed]
- Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 2016;375:819-29. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016;7:10501. [Crossref] [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [Crossref] [PubMed]
- FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. Available online: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm560040.htm
- McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463-9. [Crossref] [PubMed]
- Khunger M, Hernandez AV, Pasupuleti V, et al. Programmed Cell Death 1 (PD-1) Ligand (PD-L1) Expression in Solid Tumors As a Predictive Biomarker of Benefit From PD-1/PD-L1 Axis Inhibitors: A Systematic Review and Meta-Analysis. JCO Precision Oncology 2017;1:1-15. [Crossref]
- Peng W, Chen JQ, Liu C, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov 2016;6:202-16. [Crossref] [PubMed]
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. [Crossref] [PubMed]


