Tissue-nonspecific alkaline phosphatase: a promising target for pseudoxanthoma elasticum therapy
In mammals, biominerals consist of calcium and phosphate which form hydroxyapatite. Complex molecular and cellular mechanisms have evolved permitting crystallization of calcium and phosphate (i.e., forming hydroxyapatite) only at specific sites of the body. Pyrophosphate (PPi) is a central factor in the prevention of pathological calcium-phosphate precipitation in soft peripheral tissues [for a recent review see (1)].
Pseudoxanthoma elasticum (PXE, OMIM 264800) is a slowly progressing inherited calcification disease with clinical manifestations in the skin, eyes and in the tunica media of arterial blood vessels. The prevalence of PXE is estimated at 1:25,000–100,000. PXE severely affects the quality of life: almost all patients eventually develop eye lesions and a subset of patients suffers from cardiovascular problems [for a recent review see (2,3)]. Due to calcification of the Bruch’s membrane of the eye what is accompanied with angioid streaks and often results in neovascularization, the most severe clinical symptom in PXE is the loss of central vision in late-stage of the disease. The first symptoms affect the skin, where yellow papules appear on the neck. Later the skin looses elasticity and gets wrinkled. Mineralized lesions in small and medium-sized artery walls are typical and peripheral artery disease causing intermittent claudication is often reported by PXE patients. Mutations in ABCC6 cause pseudoxanthoma elasticum.
PXE is generally considered as a “metabolic disease”, this assumption is based on the following results: (I) the protein, ABCC6, is an organic anion transporter what is not expressed in the tissues of organs attacked by calcification, but it is highly expressed in the liver, and to a much lesser extent in the kidney and the intestines. This observation was the basis of the early hypothesis that an unknown metabolite (or its precursor) is transported from the liver into the circulation thus preventing connective tissue/soft tissue ectopic calcification; (II) experiments performed in the Uitto lab supported the above hypothesis: parabiotic connection of the circulation of Abcc6–/– and wt mice prevented calcification to a large extent in the KO animal (4). Transplantation of skin grafts between wt and KO mice resulted in prevention of calcification of the graft from the KO onto the wt, while the skin transplant from wt onto KO has been calcified (5); (III) metabolome screening by the van der Wetering laboratory provided evidence that ABCC6 is capable to transport (more accurately to facilitate the transport) of mono-, di- and trinucleotides, including ATP. The same lab showed that ATP is released from hepatocytes on an ABCC6-mediated manner in the liver microcirculation where it is promptly converted to AMP and PPi by the ectoenzyme ectonucleotide pyrophosphatase phosphodiesterase 1, ENPP1 (6,7). They also showed that the plasma level of PPi in PXE patients and in the Abcc6–/– mice is approximately 40% of the normal. A massive PPi efflux from the liver by perfusion was detected, the efflux was highly reduced when Abcc6–/– animals were studied. On the same line, lower PPi release from Abcc6–/– hepatocytes kept in sandwich culture was shown when compared to the wild type. These findings led to the conclusion that the main source of circulating PPi is the liver.
ABCC6 is a transmembrane transporter, and is localized to the sinusoidal side of hepatocytes (i.e., in the basolateral membrane compartment) (8,9). ENPP1 is also present in the liver as a membrane-attached protein. AMP, generated by ENPP1 is further cleaved to Pi and adenosine by the ecto 5’-nucleotidase, NT5E (CD73). Adenosine is a potent inhibitor of tissue non-specific alkaline phosphatase TNAP (encoded by ALPL). Reduction in adenosine level has been suggested to cause the calcification of large arteries (10) due to increased TNAP activity. TNAP hydrolyses PPi; this explains why NT5E-deficiency leads indirectly to reduction of PPi levels. Indeed, it was shown that mice lacking Nt5e have reduced plasma PPi due to the higher TNAP activity because of the lack of adenosine, leading to the degradation of extracellular PPi (11).
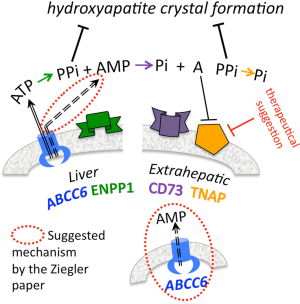
Inactivating mutations in the genes encoding enzymes involved in PPi homeostasis result in rare hereditary calcification disorders: in addition to the ABCC6–PXE relationship, mutations in ENPP1 are the genetic background of Generalized Arterial Calcification of Infancy (GACI, OMIM 208000), while Calcification of Joints and Arteries; CALJA, alternative name: Arterial Calcification due to CD73 Deficiency, ACDC (OMIM 211800) is caused by mutations in NT5E. The schematic representation of the above anti-calcification network is shown of Figure 1.
No specific treatment is available for halting of ectopic mineralization in PXE. However, in the treatment of ocular complications utilizing vascular endothelial growth factor antagonists has been proven to be a successful intervention which prevents neovascularization and generally helps to preserve vision (12). As the most severe complication of PXE is the loss of vision, this approach provides a great help for PXE patients.
The paper we discuss here brings two major messages: the first is related to the generally accepted “metabolic mechanism”, while the second is a novel idea for intervention of calcification in PXE, thus opening avenues for a new direction of therapy.
The authors carried out several knock-out experiments to demonstrate the role of Abcc6, Enpp1 and Nt5e genes in soft tissue calcification. They concluded from these experiments that all three genes participate in the inhibition of ectopic calcification. They suggest a mechanism according to which ABCC6 and CD73 cooperate downstream of ENPP1 to inhibit TNAP expression thus providing sufficient PPi levels to circumvent soft tissue calcification. As it is written in the paper: “we provide both genetic and biochemical studies that suggest a role for ABCC6 distal to the degradation of ATP to AMP and PPi by ENPP1”. However, to fully understand the molecular mechanism of the observed phenomenon, further experiments should be carried out and they don’t exclude the validity of the original model either. This led us to propose a mechanistic model what we graphically summarize on Figure 1, and indicate the suggested hypothetical extensions of the original model.
By performing partial knock-out experiments Ziegler and coworkers also showed, that several tissues participate in the Abcc6 dependent ectopic calcification. Indeed, they observe that when Abcc6 is deleted only from the major site of expression (from the liver, under otherwise wild-type conditions), the ectopic calcification phenotype is much less prominent although still present compared to complete Abcc6 knock out animal (i.e., in the case of Abcc6–/–). This strongly suggests that other tissues, in which Abcc6 is expressed to a much lower extent than in the liver, e.g., the kidney or the intestines, also play role in the pathomechanism of soft tissue calcification at least in conditions when ABCC6 is absent from the liver. In such a case these or other tissues might partially rescue the phenotype either by upregulating their own Abcc6 expression or by other, unknown mechanisms. The local events described elegantly in the Ziegler paper are important and complement well the primary systemic events associated to the liver. This may be supported by the fact that PPi supplementation therapies in Abcc6–/– mice or restoring ABCC6 activity exclusively in the liver led to 60–80% inhibition of calcification in the same mouse model Ziegler et al. used (13-16).
The paper convincingly raised the therapeutic potential of a TNAP inhibitor (SBI-425) in the Abcc6–/– mouse model. This is a very important discovery. They state that “although TNAP inhibition does not reverse existing calcification in this experimental context, it can prevent progression of the phenotype..”. This result is in line with other therapeutic options suggested very recently, as we discuss in detail later in the present editorial. The above result enforces that TNAP is an important calcification mediator and raises the possibility that in other connective tissue calcification disorders (e.g., in CD73 deficiency) it may be considered as a therapeutic target. Ziegler and colleagues found that plasma PPi level remained unchanged (i.e., did not increase) upon SBI-425 treatment although the plasma TNAP activity was decreased. This is somewhat surprising in the light of previous results demonstrating significantly reduced PPi concentration in the plasma of Nt5e–/– mice (11). As we discussed above, the lack of NT5E leads to higher TNAP activity thus resulting higher rate of PPi hydrolysis (PPi elimination). One would expect that inhibiting PPi hydrolytic activity of TNAP should increase plasma PPi concentration. However, Ziegler et al. found no such increase. This apparent discrepancy requires further studies to be resolved.
In the current year there was a rich “harvest” of preclinical results toward potential systemic intervention (i.e., not only for the eye symptoms) in PXE. Each of them based on the normalization of PPi level—like in the paper we discuss in which inhibition of an enzyme (TNAP), which catalyses PPi hydrolysis is the basis of the therapeutical suggestions.
We list here those preclinical studies, which used in vivo approaches (i.e., Abcc6–/– mice) and reported successful inhibition of calcification, on a similar manner as the Ziegler paper.
The Le Saux group demonstrated that a single daily injection of PPi to Abcc6–/– mice over several months prevented the development of spontaneous calcification (14). This raises the possibility that daily one transient increase of plasma PPi level may have preventive effect. However, due to the necessity to treat patients life-long, oral administration of PPi would be the preferential treatment. As phosphatases are abundantly present in the gut (17) it has been always claimed that orally administered PPi cannot appear in the circulation (i.e., its bioavailability is practically zero) and therefore it is not effective in inhibiting connective tissue calcification (1). In spite of the general assumption, we demonstrated significant inhibition of calcification in two different well-characterized mouse models of ectopic calcification disorders, PXE and GACI, when the animals were treated with PPi orally. The observed transient elevation of PPi in plasma found in healthy human volunteers indicates that in PXE patients PPi levels may be transiently raised to the physiological level with the dosage used. As PPi is considered to be non-toxic this option may have great clinical potentials.
Most mutations in ABCC6 are missense, and many of these mutations preserve transport activity but cause intracellular retention (18,19). This year it has been published that chemical chaperone 4-phenylbutyrate (4-PBA) not only promotes the maturation of missense ABCC6 mutants to the plasma membrane but restores the physiological function of selected ABCC6 missense mutants expressed in the liver of living Abcc6–/– mice resulting in calcification inhibition (13). This study identifies the treatments based on the repurposing use of 4-PBA as a promising strategy for allele-specific therapy in PXE.
The remarkable finding of Ziegler et al., that inhibition of TNAP is also effective in attenuating calcification in the animal model of PXE is a promising alternative approach. The above listed options fuel the optimism that treatments targeting the calcification symptoms of PXE are foreseeable. It is also possible that combination of the above interventions will be the most effective.
Acknowledgements
Funding: This work was supported by Hungarian grants OTKA 114336 and VKSz14-1-2015-0155 and by the PXE International to A Váradi, by the J. William Fulbright Scholar grant to F Szeri. and by a grant from Angers University to T Arányi.
Footnote
Conflicts of Interest: A Váradi and F Szeri filed a patent “Oral pyrophosphate for use in reducing tissue calcification” to the Netherland Patent Office (P32885NL00/RKI). The other authors have no conflicts of interest to declare.
References
- Orriss IR, Arnett TR, Russell RG. Pyrophosphate: a key inhibitor of mineralisation. Curr Opin Pharmacol 2016;28:57-68. [Crossref] [PubMed]
- Germain DP. Pseudoxanthoma elasticum. Orphanet J Rare Dis 2017;12:85. [Crossref] [PubMed]
- Favre G, Laurain A, Aranyi T, et al. The ABCC6 Transporter: A New Player in Biomineralization. Int J Mol Sci 2017;18:E1941. [Crossref] [PubMed]
- Jiang Q, Oldenburg R, Otsuru S, et al. Parabiotic heterogenetic pairing of Abcc6-/-/Rag1-/- mice and their wild-type counterparts halts ectopic mineralization in a murine model of pseudoxanthoma elasticum. Am J Pathol 2010;176:1855-62. [Crossref] [PubMed]
- Jiang Q, Endo M, Dibra F, et al. Pseudoxanthoma elasticum is a metabolic disease. J Invest Dermatol 2009;129:348-54. [Crossref] [PubMed]
- Jansen RS, Kucukosmanoglu A, de Haas M, et al. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Natl Acad Sci U S A 2013;110:20206-11. [Crossref] [PubMed]
- Jansen RS, Duijst S, Mahakena S, et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Vasc Biol 2014;34:1985-9. [Crossref] [PubMed]
- Scheffer GL, Hu X, Pijnenborg AC, et al. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest 2002;82:515-8. [Crossref] [PubMed]
- Pomozi V, Le Saux O, Brampton C, et al. ABCC6 is a basolateral plasma membrane protein. Circ Res 2013;112:e148-51. [Crossref] [PubMed]
- St. Hilaire C, Ziegler SG, Markello TC, et al. NT5E Mutations and Arterial Calcifications. N Engl J Med 2011;364:432-42. [Crossref] [PubMed]
- Li Q, Price TP, Sundberg JP, et al. Juxta-articular joint-capsule mineralization in CD73 deficient mice: similarities to patients with NT5E mutations. Cell Cycle 2014;13:2609-15. [Crossref] [PubMed]
- Verbraak FD. Antivascular endothelial growth factor treatment in pseudoxanthoma elasticum patients. Dev Ophthalmol 2010;46:96-106. [Crossref] [PubMed]
- Pomozi V, Brampton C, Szeri F, et al. Functional Rescue of ABCC6 Deficiency by 4-Phenylbutyrate Therapy Reduces Dystrophic Calcification in Abcc6-/- Mice. J Invest Dermatol 2017;137:595-602. [Crossref] [PubMed]
- Pomozi V, Brampton C, van de Wetering K, et al. Pyrophosphate Supplementation Prevents Chronic and Acute Calcification in ABCC6-Deficient Mice. Am J Pathol 2017;187:1258-72. [Crossref] [PubMed]
- Dedinszki D, Szeri F, Kozák E, et al. Oral administration of pyrophosphate inhibits connective tissue calcification. EMBO Mol Med 2017;9:1463-70. [Crossref] [PubMed]
- Brampton C, Aherrahrou Z, Chen LH, et al. The level of hepatic ABCC6 expression determines the severity of calcification after cardiac injury. Am J Pathol 2014;184:159-70. [Crossref] [PubMed]
- Ferguson A, Watson WC, Maxwell JD, et al. Alkaline phosphatase levels in normal and diseased small bowel. Gut 1968;9:96-8. [Crossref] [PubMed]
- Pomozi V, Brampton C, Fulop K, et al. Analysis of pseudoxanthoma elasticum-causing missense mutants of ABCC6 in vivo; pharmacological correction of the mislocalized proteins. J Invest Dermatol 2014;134:946-53. [Crossref] [PubMed]
- Le Saux O, Fulop K, Yamaguchi Y, et al. Expression and in vivo rescue of human ABCC6 disease-causing mutants in mouse liver. PLoS One 2011;6:e24738. [Crossref] [PubMed]


