Antibody-drug conjugate targeting protein tyrosine kinase 7, a receptor tyrosine kinase-like molecule involved in WNT and vascular endothelial growth factor signaling: effects on cancer stem cells, tumor microenvironment and whole-body homeostasis
Introduction
Cancer stem cells (CSCs) and tumor-initiating cells (TICs) are subpopulations of tumor cells that are defined by self-renewal and multi-differentiation potentials and in vivo tumorigenicity potential, respectively (1,2). Because TICs de-differentiate into CSCs owing to their plasticity, and then, CSCs give rise to proliferating or differentiated bulk tumor cells for tumor initiation, CSCs and TICs are almost similar concepts in the field of cancer biology. CSCs/TICs have been identified in a variety of human cancers, such as breast cancer (3), colorectal cancer (4), gastric cancer (5) and lung cancer (6), and are characterized by cancer type- or subtype-specific CSC markers, such as BMI1 (7), CD133 (PROM1) (8), LGR5 (GPR49) (9) and PTK7 (2,10).
CSCs acquire more malignant features during tumor progression through accumulation of genetic and epigenetic alterations (1). CSCs undergo epithelial-to-mesenchymal transition (EMT) to promote invasion and metastasis, whereas CSCs undergo dormancy to survive cancer therapy for later relapse. Small-molecule inhibitors or antibody-based drugs that target receptor tyrosine kinases (RTKs) have been contributing to improved prognosis of cancer patients; however, drug resistance and recurrence are serious issues that are difficult to avoid. Because CSCs play key roles in tumor progression, therapeutic resistance and tumor recurrence, development of CSC-targeted therapy is mandatory to further improve quality-of-life and survival of cancer patients (1).
Human/humanized monoclonal antibodies (mAbs), such as anti-HER2 mAb (trastuzumab), anti-PD-1 mAbs (pembrolizumab and nivolumab), anti-PD-L1 mAbs (atezolizumab, avelumab and durvalumab) and anti-vascular endothelial growth factor (VEGF) mAb (bevacizumab), are antibody-based drugs that are approved for the treatment of cancer patients, whereas antigen-drug conjugate (ADC), bispecific antibody (bsAb) and chimeric antigen receptor-engineered T (CAR-T) cells are structurally modified antibody-based drugs (11-14). Recently, Damelin and colleagues developed an anti-protein tyrosine kinase 7 (PTK7) ADC, PF-06647020, that targets CSCs and tumor microenvironment to induce direct and indirect anti-tumor effects, respectively (2). Here, structure and physiological functions of PTK7 (Figure 1) as well as human diseases related to PTK7 will be presented, and then mechanisms-of-action and perspectives of the PTK7-targeted ADC will be discussed.
Structure and functions of PTK7
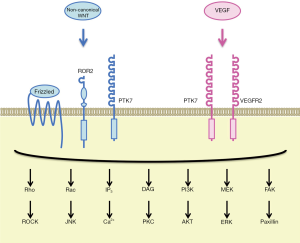
PTK7 is an RTK family member that was originally cloned as characterized as colon carcinoma kinase 4 (CCK-4) overexpressed in colon cancer (15,16). PTK7 is a single-pass transmembrane receptor with seven immunoglobulin-like domains in the extracellular region and a tyrosine kinase-like domain in the intracellular region. PTK7 undergoes sequential cleavage by ADAM metallopeptidase domain 17 (ADAM17) and γ-secretase, which leads to shedding of the extracellular region of PTK7 (PTK7-ECD) as well as release and nuclear translocation of the intracellular region of PTK7 (PTK7-ICD) (17). Although PTK7 is an atypical RTK without intrinsic tyrosine kinase activity, PTK7-dependent signaling plays key roles during embryogenesis and carcinogenesis. Involvement of PTK7 in the regulation of WNT and VEGF signaling cascades and maintenance of somatic stem cells will be described in the following part of this section.
Canonical WNT signals are transduced to the β-catenin-TCF/LEF and stabilization of proteins (STOP) signaling cascades, whereas non-canonical WNT signals are transduced to the planar cell polarity (PCP), G-protein couple receptor (GPCR) and RTK signaling cascades (18,19). PTK7 and Frizzled-7 (FZD7) function as WNT2B receptors to inhibit the canonical WNT/β-catenin signaling cascade through caveolin-dependent internalization of the WNT receptor complex (20), whereas PTK7 and ROR2 function as WNT5A receptors to activate the non-canonical WNT/PCP signaling cascades (21). Because PCP signaling pathway regulates the cellular movement as well as cellular polarity in the epithelial plane (19), germ-line point mutations in the human PTK7 gene, such as G348S, occur in patients with neural tube defects (22) and a germ-line truncation mutation in the mouse PTK7 gene gives rise to impairment of neural tube closure and disorientation of stereociliary bundles (23). PTK7 is a co-receptor of WNT family ligands that can class-switch WNT signaling to the non-canonical WNT/PCP signaling cascades.
VEGF (VEGFA) is a representative pro-angiogenic factor that transduces signals through VEGFR1 (FLT1) and VEGFR2 (KDR) receptors to promote angiogenic sprouting of endothelial cells (24). VEGFR1, VEGFR2 and PTK7 share a common domain architecture that consists of seven immunoglobulin-like domains, a transmembrane domain and a tyrosine kinase domain, although tyrosine kinase domain of PTK7 is significantly divergent from those of VEGFRs (16). PTK7, associating with VEGFR2 rather than VEGFR1 in human umbilical vein endothelial cells (HUVECs), is involved in VEGF-induced VEGFR2 phosphorylation and angiogenic sprouting of endothelial cells (25).
PTK7 is co-expressed with other stem cell markers, such as LGR5 and ASCL2, on human intestinal stem/progenitor cells, and those with higher PTK7 levels are characterized by higher self-renewal potential (26). By contrast, mouse PTK7 is relatively highly expressed on hematopoietic stem cells and multipotent progenitor cells, relatively weakly expressed on common lymphoid progenitors, but not detectable on granulocyte/macrophage and erythroid/megakaryocyte progenitors in bone marrow and mature leukocytes in peripheral blood, whereas hematopoietic stem cells in the fetal liver of PTK7 knockout mice are more quiescent and defective in homing and migration potentials (27). These facts indicate that PTK7 is involved in the maintenance of somatic stem cells, such as intestinal stem cells and hematopoietic stem cells.
Context-dependent functions of PTK7 in human cancers
Involvements of PTK7 in various types of human cancers have been investigated, because PTK7 is located at a crossroads of the WNT signaling, VEGF signaling and stem cell biology. PTK7 is upregulated in various types of human cancers, such as atypical teratoid rhabdoid tumors (ATRTs) (28), breast cancer (29), cholangiocarcinoma (30), colorectal cancer (31,32), esophageal squamous cell carcinoma (33) and gastric cancer (34).
ATRTs are rare pediatric brain tumors with poor prognosis. Proliferation and viability of ARTR cells were repressed by vatalanib targeting multiple tyrosine kinases and siRNA-mediated PTK7 knockdown, respectively (28). Motility and invasion of breast cancer cells were repressed by overexpression of kinase domain-defective PTK7 mutant, anti-PTK7 polyclonal antibody or PTK7 knockdown (29). Because PTK7 is a non-canonical WNT signaling component that is involved in activation of downstream PI3K-AKT, Rho and SRC signaling cascades (19-21), PTK7 can promote survival, motility and invasion of cancer cells through non-canonical WNT signaling activation. PTK7 upregulation is associated with poor prognosis in patients with breast cancer (29) and cholangiocarcinoma (30) as well as colorectal cancer patients in France (31).
By contrast, because non-canonical WNT signals are able to inhibit the canonical WNT/β-catenin signaling cascade (19,20), PTK7 can exert tumor suppressor function through canonical WNT/β-catenin signaling inhibition. PTK7 upregulation is associated with favorable prognosis in patients with gastric cancer (34) as well as colorectal cancer patients in China (32).
Together these facts clearly indicate that PTK7 functions as a cancer driver or tumor suppressor in a cell context-dependent manner as a result of complexity in the canonical and non-canonical WNT signaling network.
Preclinical study of PTK7-targeted ADC for cancer treatment
Damelin and colleagues hypothesized that an ADC based on anti-PTK7 mAb is able to deliver anti-cancer drugs preferentially into CSCs, because PTK7 was upregulated in TICs of patient-derived xenograft (PDX) models for non-small cell lung cancer (NSCLC), ovarian cancer (OVCA) and triple-negative breast cancer (TNBC) (2). They generated anti-PTK7 ADC, PF-06647020, with an average drug/antibody ratio of 4 through mc-valine-citrulline-PABC linker-mediated bioconjugation of microtubule inhibitor Aur0101 to humanized anti-PTK7 mAb. PF-06647020 showed in vitro cytotoxic effects on PTK7 expressing cancer cell lines H446, H661 and OVCAR3 with EC50 values of 7.6±5.0, 27.5±20.5 and 105±17 ng/mL, respectively. Intra-peritoneal injection of PF-06647020 (3 mg/kg, twice a week for four cycles) induced striking in vivo anti-tumor effects on a subset of PDXs derived from NSCLC, OVCA and TNBC (2). For example, sustained regression of OVCA PDX (OV55) and TNBC PDX (BR22) persisted for approximately 200 days after PF-06647020 treatment without subsequent recurrence, and sustained regression of NSCLC PDX (LU176) persisted for approximately 100 days after PF-06647020 treatment with subsequent recurrence. In addition, PF-06647020 decreased TIC frequency in TNBC PDX (BR13) by 5.5-fold compared with control ADC. These results indicate anti-CSC effects of PTK7-targeted ADC in preclinical model experiments (Figure 2).
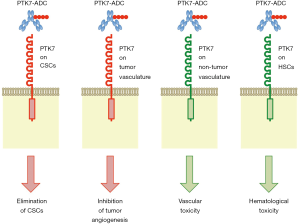
Damelin and colleagues also investigated indirect anti-tumor effects of PF-06647020 through stromal or immune cells in the tumor microenvironment, because anti-PTK7 immuno-reactivity was detected on cancer cells as well as non-cancerous cells (2). Shedding of PTK7-ECD through ADAM17-dependent cleavage (17) might in part explain stromal anti-PTK7 immuno-reactivity; however, PTK7 is expressed on HUVECs and plasmacytoid dendritic cells (pDCs) in primary tumors and peripheral blood (2). Damelin and colleagues suggested that PF-06647020 might potentiate anti-tumor immunity through targeting immune-suppressive pDCs. Because WNT signals are transduced to canonical and non-canonical WNT signaling cascades (18) and regulate anti-tumor immunity and immune evasion in a cell context-dependent manner (19), further studies on pDCs and tumor immunity are necessary to demonstrate PF-06647020-induced potentiation of anti-tumor immunity. By contrast, Damelin and colleagues showed that PF-06647020 inhibited angiogenic sprouting through its effects on endothelial cells (2). PTK7-targeted ADC elicits anti-tumor effects indirectly through stromal cells (Figure 2).
Damelin and colleagues then addressed the safety issue of PF-06647020 in primate model experiments (2). Repeated administration of PF-06647020 to cynomolgus monkeys (up to 5 mg/kg, once every 3 weeks for three cycles) induced myelosuppression, but no signs of toxicities in PTK7-expressing tissues, such as esophagus, kidney, lung and urinary bladder. Because average PF-06647020 concentration of 11.8 µg/mL in cynomolgus monkeys dosed at 5 mg/kg without no severe toxicities was higher than predicted human efficacy concentration of 2.9 to 7.0 µg/mL, PF-06647020 would have a therapeutic window in cancer patients.
Perspectives
PF-06647020 is a promising anti-cancer therapeutics and is currently in phase I clinical trial for the treatment of patients with advanced solid tumors (ClinicalTrials.gov Identifier: NCT02222922). Because CSCs (2), endothelial cells (25) and hematopoietic stem cells (27) with PTK7 expression are targets of the anti-PTK7 ADC, bleeding and pancytopenia might occur as on-target adverse effects of PF-06647020 (Figure 2). It is necessary to monitor vascular and hematological toxicities in cancer patients, especially elder patients, undergone PF-06647020 treatment.
By contrast, PF-06647020 induced regression in a subset of PDXs in preclinical model experiments (2). Because PTK7 and other non-canonical WNT signaling components are oncogenic or tumor suppressive depending on tumor origin or subtype (19-21), PTK7-targeted ADC might be beneficial only for cancer patients with oncogenic PTK7 upregulation. Companion diagnostics for patient selection should be developed to ensure a favorable benefit-risk profile of PF-06647020.
RTKs are frequently overexpressed or aberrantly activated in human cancers (1), and preferable targets of ADCs owing to accessibility of and internalization with ADC (35). HER2-targeting ADC (trastuzumab emtansine) is already approved for the treatment of breast cancer patients, whereas ADCs targeting PTK7 as well as other RTKs, such as AXL, EGFR, EPHA2, ERBB3, FGFR2, FGFR3, FLT3, KIT and MET, are under development for the treatment of cancer patients (35,36). Development of a spectrum of ADCs targeting cancer-related RTKs would contribute to the implementation of personal/precision medicine.
Acknowledgements
This work was supported in part by a grant-in-aid from M. Katoh’s Fund for the Knowledge-Base Project.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Katoh M. Therapeutics Targeting FGF Signaling Network in Human Diseases. Trends Pharmacol Sci 2016;37:1081-96. [Crossref] [PubMed]
- Damelin M, Bankovich A, Bernstein J, et al. A PTK7-targeted antibody-drug conjugate reduces tumor-initiating cells and induces sustained tumor regressions. Sci Transl Med 2017;9:eaag2611. [Crossref] [PubMed]
- D'Angelo RC, Ouzounova M, Davis A, et al. Notch reporter activity in breast cancer cell lines identifies a subset of cells with stem cell activity. Mol Cancer Ther 2015;14:779-87. [Crossref] [PubMed]
- Pan T, Xu J, Zhu Y. Self-renewal molecular mechanisms of colorectal cancer stem cells. Int J Mol Med 2017;39:9-20. [Crossref] [PubMed]
- Xu M, Gong A, Yang H, et al. Sonic hedgehog-glioma associated oncogene homolog 1 signaling enhances drug resistance in CD44(+)/Musashi-1(+) gastric cancer stem cells. Cancer Lett 2015;369:124-33. [Crossref] [PubMed]
- Lundin A, Driscoll B. Lung cancer stem cells: progress and prospects. Cancer Lett 2013;338:89-93. [Crossref] [PubMed]
- Bartucci M, Hussein MS, Huselid E, et al. Synthesis and Characterization of Novel BMI1 Inhibitors Targeting Cellular Self-Renewal in Hepatocellular Carcinoma. Target Oncol 2017;12:449-62. [Crossref] [PubMed]
- Grosse-Gehling P, Fargeas CA, Dittfeld C, et al. CD133 as a biomarker for putative cancer stem cells in solid tumours: limitations, problems and challenges. J Pathol 2013;229:355-78. [Crossref] [PubMed]
- de Sousa e Melo F, Kurtova AV, Harnoss JM, et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 2017;543:676-80. [Crossref] [PubMed]
- Asad M, Wong MK, Tan TZ, et al. FZD7 drives in vitro aggressiveness in Stem-A subtype of ovarian cancer via regulation of non-canonical Wnt/PCP pathway. Cell Death Dis 2014;5:e1346. [Crossref] [PubMed]
- Smyth MJ, Ngiow SF, Ribas A, et al. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol 2016;13:143-58. [Crossref] [PubMed]
- Ayyar BV, Arora S, O'Kennedy R. Coming-of-Age of Antibodies in Cancer Therapeutics. Trends Pharmacol Sci 2016;37:1009-28. [Crossref] [PubMed]
- Gong X, Azhdarinia A, Ghosh SC, et al. LGR5-Targeted Antibody-Drug Conjugate Eradicates Gastrointestinal Tumors and Prevents Recurrence. Mol Cancer Ther 2016;15:1580-90. [Crossref] [PubMed]
- Berger C, Sommermeyer D, Hudecek M, et al. Safety of targeting ROR1 in primates with chimeric antigen receptor-modified T cells. Cancer Immunol Res 2015;3:206-16. [Crossref] [PubMed]
- Mossie K, Jallal B, Alves F, et al. Colon carcinoma kinase-4 defines a new subclass of the receptor tyrosine kinase family. Oncogene 1995;11:2179-84. [PubMed]
- Katoh M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis Int J Mol Med 2016;38:3-15. (Review). [Crossref] [PubMed]
- Na HW, Shin WS, Ludwig A, et al. The cytosolic domain of protein-tyrosine kinase 7 (PTK7), generated from sequential cleavage by a disintegrin and metalloprotease 17 (ADAM17) and γ-secretase, enhances cell proliferation and migration in colon cancer cells. J Biol Chem 2012;287:25001-9. [Crossref] [PubMed]
- Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res 2007;13:4042-5. [Crossref] [PubMed]
- Katoh M, Katoh M. Molecular genetics and targeted therapy of WNT-related human diseases Int J Mol Med 2017;40:587-606. (Review). [PubMed]
- Martinez S, Scerbo P, Giordano M, et al. The PTK7 and ROR2 Protein Receptors Interact in the Vertebrate WNT/Planar Cell Polarity (PCP) Pathway. J Biol Chem 2015;290:30562-72. [Crossref] [PubMed]
- Berger H, Breuer M, Peradziryi H, et al. PTK7 localization and protein stability is affected by canonical Wnt ligands. J Cell Sci 2017;130:1890-903. [Crossref] [PubMed]
- Wang M, De Marco P, Merello E, et al. Role of the planar cell polarity gene Protein tyrosine kinase 7 in neural tube defects in humans. Birth Defects Res A Clin Mol Teratol 2015;103:1021-7. [Crossref] [PubMed]
- Lu X, Borchers AG, Jolicoeur C, et al. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature 2004;430:93-8. [Crossref] [PubMed]
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer 2013;13:871-82. [Crossref] [PubMed]
- Shin WS, Na HW, Lee ST. Biphasic effect of PTK7 on KDR activity in endothelial cells and angiogenesis. Biochim Biophys Acta 2015;1853:2251-60. [Crossref] [PubMed]
- Jung P, Sommer C, Barriga FM, et al. Isolation of Human Colon Stem Cells Using Surface Expression of PTK7. Stem Cell Reports 2015;5:979-87. [Crossref] [PubMed]
- Lhoumeau AC, Arcangeli ML, De Grandis M, et al. Ptk7-Deficient Mice Have Decreased Hematopoietic Stem Cell Pools as a Result of Deregulated Proliferation and Migration. J Immunol 2016;196:4367-77. [Crossref] [PubMed]
- Messerli SM, Hoffman MM, Gnimpieba EZ, et al. Therapeutic Targeting of PTK7 is Cytotoxic in Atypical Teratoid Rhabdoid Tumors. Mol Cancer Res 2017;15:973-83. [Crossref] [PubMed]
- Gärtner S, Gunesch A, Knyazeva T, et al. PTK 7 is a transforming gene and prognostic marker for breast cancer and nodal metastasis involvement. PLoS One 2014;9:e84472. [Crossref] [PubMed]
- Jin J, Ryu HS, Lee KB, et al. High expression of protein tyrosine kinase 7 significantly associates with invasiveness and poor prognosis in intrahepatic cholangiocarcinoma. PLoS One 2014;9:e90247. [Crossref] [PubMed]
- Lhoumeau AC, Martinez S, Boher JM, et al. Overexpression of the Promigratory and Prometastatic PTK7 Receptor Is Associated with an Adverse Clinical Outcome in Colorectal Cancer. PLoS One 2015;10:e0123768. [Crossref] [PubMed]
- Tian X, Yan L, Zhang D, et al. PTK7 overexpression in colorectal tumors: Clinicopathological correlation and prognosis relevance. Oncol Rep 2016;36:1829-36. [Crossref] [PubMed]
- Shin WS, Hong Y, Lee HW, et al. Catalytically defective receptor protein tyrosine kinase PTK7 enhances invasive phenotype by inducing MMP-9 through activation of AP-1 and NF-κB in esophageal squamous cell carcinoma cells. Oncotarget 2016;7:73242-56. [PubMed]
- Lin Y, Zhang LH, Wang XH, et al. PTK7 as a novel marker for favorable gastric cancer patient survival. J Surg Oncol 2012;106:880-6. [Crossref] [PubMed]
- Beck A, Goetsch L, Dumontet C, et al. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov 2017;16:315-37. [Crossref] [PubMed]
- Beck A, Terral G, Debaene F, et al. Cutting-edge mass spectrometry methods for the multi-level structural characterization of antibody-drug conjugates. Expert Rev Proteomics 2016;13:157-83. [Crossref] [PubMed]


