Drugs targeting protease-activated receptor-4 improve the anti-thrombotic therapeutic window
Introduction
Antiplatelet agents are the main pharmacotherapy for arterial thrombosis prevention and are central in the management of cardiovascular conditions such as myocardial infarction, transient ischaemic attack, and coronary and peripheral artery diseases. Yet despite their long history and extensive clinical use, antiplatelet agents appear to have reached a disappointingly low therapeutic ceiling-predominantly due to the narrow therapeutic window afforded by strategies targeting platelet function. Platelets are critical for normal hemostasis as well as pathological thrombosis. Inhibiting platelet function for protective benefit without causing unwanted bleeding limits the efficacy of current antiplatelet drugs. Aspirin and the thienopyridine class of drugs (e.g., clopidogrel, prasugrel, ticagrelor) are by far the most commonly prescribed antiplatelet agents, yet prevent just 15 and 17% of lethal cardiovascular events respectively (1,2). Combination therapy provides a marginal increase in efficacy (~7%), but also increases the risk of bleeding (2). More potent antiplatelet drugs, such as the glycoprotein αIIbβ3 inhibitors, carry even more bleeding risk and are thereby limited to acute use settings such as periprocedural percutaneous coronary intervention (3,4). Therefore, the search to identify antiplatelet drugs that increase the therapeutic window of antithrombotic therapy continues. A recent study by Wong et al. (5) provides compelling evidence that targeting the platelet thrombin receptor, PAR4, may achieve this goal.
In the setting of thrombosis, platelets are activated by a combination of endogenous agonists, some of which are blocked by existing drugs. For example, aspirin prevents production of the platelet activator thromboxane A2 while the thienopyridines block the major platelet ADP receptor, P2Y12. Thrombin is the most potent platelet activator, which it achieves predominantly via two cell surface GPCRs, protease-activated receptor 1 (PAR1) and PAR4. PAR1 has greater affinity for thrombin than PAR4 and has therefore been the focus of drug development targeting thrombin-induced platelet activation. The first PAR1 antagonist, vorapaxar, was approved by the US FDA in 2014 for the prevention of thrombotic events in patients with a history of myocardial infarction or peripheral artery disease. Yet because it must be administered in addition to standard-of-care antiplatelet therapy (aspirin and/or a thienopyridine), vorapaxar provides only limited therapeutic benefit to a small group of patients without significantly increasing major bleeding (6,7). In line with the clinical experience of other combination antiplatelet therapies, the narrow therapeutic window of vorapaxar in the presence of standard-of-care antiplatelet drugs has translated to limited clinical utility. As a result, there has been much renewed interest in targeting the ‘second’ platelet thrombin receptor, PAR4, for antithrombotic therapy. Although previous studies have rationalised PAR4 as a viable antithrombotic target (8-11), the work by Wong and colleagues expands on this to describe the development of a potent and specific small molecule PAR4 antagonist with a markedly improved therapeutic window over one standard antiplatelet drug (clopidogrel) in a preclinical model.
Discovery of a potent and specific small molecule PAR4 antagonist
In proof-of-concept work that supports previous studies (8-11), the team at BMS first used function-blocking anti-PAR4 antibodies in a guinea pig model to show in vivo anti-thrombotic efficacy and relative safety of selective PAR4 blockade. To shift to the highly desired small molecule approach, they then embarked on an impressive drug discovery program. The unique activation mechanism of PARs has provided a major hurdle for the development of efficacious antagonists. Thrombin cleavage of PARs reveals an endogenous tethered ligand which then binds to and self-activates the receptor. Therefore, antagonists must overcome an agonist that is intrinsic to the receptor and presumably has considerable steric advantage. Wong and colleagues screened a library of over 1 million compounds to identify a lead candidate that was then subject to iterative rounds of medicinal chemistry and testing to result in BMS-986120—a potent and selective PAR4 antagonist with impressive oral bioavailability and antithrombotic efficacy (Table 1).
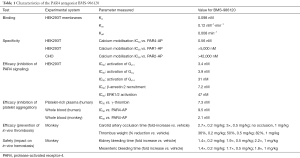
Full table
Selective inhibition of PAR4 over PAR1 has been elusive in previous efforts developing small molecule PAR4 antagonists. For example, the indazole-derivative YD-3 (12) and its derivative ML354 (13) exhibit cross-reactivity toward PAR1 (12,13). Here though, BMS-986120 demonstrated specificity in both HEK293 cells transfected with PAR4 and human platelets, with no effect observed on platelet activation by a PAR1 activating peptide, collagen, ADP, or thromboxane A2 (Table 1). To demonstrate efficacy of BMS-986120, the authors examined inhibitory profiles of platelet aggregation against two isoforms of thrombin—α and γ, thought to preferentially activate PAR1 and PAR4 respectively. BMS-986120 effectively suppressed platelet aggregation in response to γ-thrombin but required concomitant PAR1 inhibition to do so against α-thrombin. Whether complete blockade of thrombin-induced platelet activation will be required for effective antithrombotic therapy, or whether partial inhibition will be sufficient, remains to be determined.
For PAR antagonists to be efficacious against endogenous enzymatic activation of the receptor by thrombin, they must exhibit strong binding affinity. Yet in the clinical context it is highly desirable for an anti-platelet agent to have the potential to be rapidly reversed should any unwanted bleeding challenges occur. Wong et al. used (3H)-BMS-986120 binding to PAR4-expressing cell membranes to reveal that BMS-986120 is a high affinity and reversible binder of PAR4 (Table 1). In studies performed on platelets isolated from monkeys dosed with BMS-986120, this translated to normalised aggregation 24 h after a single dose of 0.2 mg/kg. A more detailed time course will be required to determine the half-life of BMS-986120. Although the dissociation constant is relatively fast, competition studies would be useful to demonstrate whether there was potential for an antidote if required. Regardless, the pharmacodynamic profile is considerably advantageous in comparison to other antiplatelet drugs. For example, platelet inhibition by the PAR1 antagonist vorapaxar is retained 4–8 weeks after a single loading dose in humans (14) while aspirin and clopidogrel are both irreversible protein modifiers with long-term effects.
Most importantly though, BMS-986120 appeared to provide an impressive therapeutic window, with a single oral dose of BMS-986120 providing marked antithrombotic effects and a low bleeding profile in a series of in vivo models in the cynomolgus monkey (Table 1). Appropriate examination of in vivo platelet PAR function is limited to primates since the traditional small animal models (e.g., mice, rats, guinea-pigs, rabbits, dogs) have a different PAR expression profile to that of humans. Therefore, Wong and colleagues used an electrolytic model of carotid artery thrombosis in the cynomolgus monkey for preclinical evaluation of their PAR4 antagonist. BMS-986120 alone prevented occlusive thrombus formation at the highest dose (1 mg/kg) and significantly reduced thrombosis at lower doses (0.2–0.5 mg/kg). Hemostasis, measured either by kidney or mesenteric bleeding time, was increased by just over 2-fold at the 1 mg/kg dose and much less at the lower doses. Importantly, when this dose-response of BMS-986120 on hemostasis and thrombosis was compared directly with that of clopidogrel, there was a clear separation provided by BMS-986120 that was not evident with clopidogrel. At doses of these two agents that caused equivalent anti-thrombotic effects, markedly more bleeding was observed with clopidogrel compared with BMS-986120. For example, at a dose that caused a 50% reduction in thrombus weight, clopidogrel induced a 7.3- to 8.1-fold increase in bleeding compared with a 1.7- to 1.9-fold increase for BMS-986120. This was more pronounced at doses that caused a near 100% reduction in thrombus weight, with clopidogrel inducing a >10-fold increase in bleeding versus a 1.8- to 2.2-fold increase for BMS-986120. Given this stark difference, it would be interesting to known how the therapeutic window changed when used in combination with aspirin, P2Y12 antagonists and/or vorapaxar.
A potential mechanism for an improved therapeutic window
How is it that PAR4 inhibition provides such strong separation between impacting on thrombosis and hemostasis? One clue comes from recent work indicating that PAR4 performs distinct functions to other key platelet receptors. PAR4 activation elicits a slower, but significantly more sustained, intracellular calcium response than that elicited by PAR1 (15). This prolonged calcium signal mediates later-stage platelet activation events, such as the platelet procoagulant response involving phosphatidylserine exposure on the platelet membrane and consequent assembly of coagulation factors leading to thrombin generation and fibrin formation. Indeed, selective inhibition of PAR4 but not PAR1 significantly inhibits thrombin activity and fibrin deposition in human thrombi ex vivo (8). One explanation for the improved therapeutic window of BMS-986120 reported by Wong et al. is that PAR4 inhibition is blocking platelet function at a distinct time and place to all existing approaches.
What does the future hold for PAR4 antagonists?
BMS-986120 was evaluated in a phase 1 dosing study, yet despite efficacy and a lack of adverse events no phase 2 studies of this compound were undertaken. Rather, BMS are investigating the related compound, BMS-986141, which also underwent a phase 1 study (NCT02341638) and a subsequent phase 2 trial for the prevention of mini-stroke (NCT02671461). The trial (A Phase 2, Placebo Controlled, Randomized, Double-Blind, Parallel-Arm Study to Evaluate Efficacy and Safety of BMS-986141 For the Prevention of Recurrent Brain Infarction in Subjects Receiving Acetylsalicylic Acid Following Acute Ischemic Stroke or Transient Ischemic Attack) had a primary efficacy endpoint of a composite of symptomatic ischemic stroke or unrecognized brain infarction, and a primary safety endpoint of a composite of adjudicated major bleeding and adjudicated clinically relevant non-major bleeding during the treatment period. It was completed in April 2017 but has not yet been reported.
It is far too early to predict the likely clinical success and/or usefulness of PAR4 antagonists, and several key questions remain. How well will PAR4 antagonism combine with current standard-of-care agents? This is a central point, since any trial will be conducted in the presence of standard-of-care, which frequently involves dual antiplatelet therapy. With the PAR1 antagonist vorapaxar, for example, the increased bleeding observed is believed to be due to poor compatibility with clopidogrel. Indeed, sub-study analyses show no additional bleeding in patients receiving aspirin plus vorapaxar versus those receiving aspirin alone (6,16). Here, it is interesting to note that BMS chose to investigate a patient group being treated with aspirin alone in its first phase 2 trial of its lead PAR4 antagonist.
What specific indications will be best served by a PAR4 antagonist? Again, sub-study analyses of the vorapaxar trials may provide pointers. These trials showed the most efficacy in reducing the rate of spontaneous myocardial infarction as well as in prevention of vascular complications associated with peripheral artery disease. This is perhaps unsurprising given the well-known role of thrombin generation in acute myocardial infarction, particularly in patients with a background of unstable angina and/or coronary artery disease (17). Whether PAR4 antagonism will similarly demonstrate superior efficacy in these clinical situations where thrombin-induced platelet activation are implicated is an obvious place to start for future clinical trials.
Finally, one emerging issue for PAR4 antagonism is that of population genetics. Recent studies have revealed a commonly expressed genetic variant of PAR4 (rs773902; encoding either Ala120 or Thr120) that appears to significantly alter receptor pharmacology. Specifically, the Thr120 PAR4 variant, expressed in 20–80% of people depending on the population, renders the receptor hyper-sensitive to agonists and hypo-sensitive to antagonists (18,19). The mechanism behind this change in PAR4 pharmacology remains unknown, as does whether all PAR4 antagonists, including BMS-986120 and BMS-986141, will be similarly affected. Studies directly addressing these points will be critical in determining whether the approach proposed by Wong et al. will afford consistent antithrombotic benefit across the population.
Conclusions
The recent preclinical study by Wong et al. (5) details the development and preclinical evaluation of the first PAR4 antagonist to enter a clinical trial and represents a potentially important breakthrough in the treatment of arterial thrombosis. While further insights are still to be gained regarding the utility of PAR4 antagonism in clinical settings, this study has contributed an important reagent to help study this previously under-appreciated platelet activation mechanism, and has identified a potentially useful approach for the safe and effective prevention of arterial thrombosis.
Acknowledgements
This work was supported by grants to JR Hamilton from the National Health & Medical Research Council of Australia and the CASS Foundation. JR Hamilton is a Future Fellow of the Australian Research Council. The authors thank L Hodge for exceptional contributions over his career.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86. [Crossref] [PubMed]
- French SL, Arthur JF, Tran HA, et al. Approval of the first protease-activated receptor antagonist: Rationale, development, significance, and considerations of a novel anti-platelet agent. Blood Rev 2015;29:179-89. [Crossref] [PubMed]
- Scarborough RM, Kleiman NS, Phillips DR. Platelet glycoprotein IIb/IIIa antagonists. What are the relevant issues concerning their pharmacology and clinical use? Circulation 1999;100:437-44. [Crossref] [PubMed]
- Kastrati A, Mehilli J, Neumann FJ, et al. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA 2006;295:1531-8. [Crossref] [PubMed]
- Wong PC, Seiffert D, Bird JE, et al. Blockade of protease-activated receptor-4 (PAR4) provides robust antithrombotic activity with low bleeding. Sci Transl Med 2017;9:eaaf5294. [Crossref] [PubMed]
- Tricoci P, Huang Z, Held C, et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med 2012;366:20-33. [Crossref] [PubMed]
- Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012;366:1404-13. [Crossref] [PubMed]
- French SL, Arthur JF, Lee H, et al. Inhibition of protease-activated receptor 4 impairs platelet procoagulant activity during thrombus formation in human blood. J Thromb Haemost 2016;14:1642-54. [Crossref] [PubMed]
- Lee H, Sturgeon SA, Mountford JK, et al. Safety and efficacy of targeting platelet proteinase-activated receptors in combination with existing anti-platelet drugs as antithrombotics in mice. Br J Pharmacol 2012;166:2188-97. [Crossref] [PubMed]
- Sambrano GR, Weiss EJ, Zheng YW, et al. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature 2001;413:74-8. [Crossref] [PubMed]
- Covic L, Misra M, Badar J, et al. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat Med 2002;8:1161-5. [Crossref] [PubMed]
- Wu CC, Hwang TL, Liao CH, et al. Selective inhibition of protease-activated receptor 4-dependent platelet activation by YD-3. Thromb Haemost 2002;87:1026-33. [PubMed]
- Wen W, Young SE, Duvernay MT, et al. Substituted indoles as selective protease activated receptor 4 (PAR-4) antagonists: Discovery and SAR of ML354. Bioorg Med Chem Lett 2014;24:4708-13. [Crossref] [PubMed]
- Kosoglou T, Reyderman L, Tiessen RG, et al. Pharmacodynamics and pharmacokinetics of the novel PAR-1 antagonist vorapaxar (formerly SCH 530348) in healthy subjects. Eur J Clin Pharmacol 2012;68:249-58. [Crossref] [PubMed]
- Covic L, Gresser AL, Kuliopulos A. Biphasic kinetics of activation and signaling for PAR1 and PAR4 thrombin receptors in platelets. Biochemistry 2000;39:5458-67. [Crossref] [PubMed]
- Mahaffey KW, Huang Z, Wallentin L, et al. Association of aspirin dose and vorapaxar safety and efficacy in patients with non-ST-segment elevation acute coronary syndrome (from the TRACER Trial). Am J Cardiol 2014;113:936-44. [Crossref] [PubMed]
- Merlini PA, Bauer KA, Oltrona L, et al. Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation 1994;90:61-8. [Crossref] [PubMed]
- Edelstein LC, Simon LM, Montoya RT, et al. Racial differences in human platelet PAR4 reactivity reflect expression of PCTP and miR-376c. Nat Med 2013;19:1609-16. [Crossref] [PubMed]
- Edelstein LC, Simon LM, Lindsay CR, et al. Common variants in the human platelet PAR4 thrombin receptor alter platelet function and differ by race. Blood 2014;124:3450-8. [Crossref] [PubMed]


