Diamonds are for men, too
Introduction
The results of a simple in vitro experiment presented 2016 in an editorial (1) were commented in Science (2) and later in three editorials (3-5). What makes those results to be interesting? Is it the finding that the progressive motility of human spermatozoa increased by 300% after one hour contact with diamond Petri dishes, when compared to standard polystyrene Petri dishes? Is it the additionally reported beneficial effect of near infrared (NIR) light on the performance of the spermatozoa? Undoubtedly, the findings (1) suffice to revolutionize in vitro fertilization (IVF) routines, one day: (I) by safeguarding a critical microenvironment—the interfacial zone between cell and Petri dish—which on diamond is virtually free of a reactive oxygen species (ROS) and in which spermatozoa have a higher probability to fertilize oocytes and (II) by using NIR light to fill up mitochondrial adenosine triphosphate (ATP) reservoirs. However, the results presented in (1) are in no way restricted to IVF. Probably, their validity can be extended to in vitro tests in general and thus practically exploited in life sciences and medicine.
For a justification of the aforementioned general validity postulate, it is instructive to review the basic cellular components and physiological properties in the three editorials (3-5) whose interplay is considered by their authors to be relevant for the discussion/interpretation of the in vitro experiments performed in the diamond and polystyrene Petri dishes, respectively (1). Batsanov et al.: interfacial water viscosity, mitochondria, electrons, ATP, ROS. Lanzafame: water, mitochondria, ATP, ROS, cytochrome c oxidase. Försterling et al.: interfacial water viscosity, mitochondria, ATP, ROS. Clearly, these components/properties are not typical for spermatozoa. Actually, they are so fundamental that there is no cell containing mitochondria where their interaction would not control elementary biological processes, including cell survival and apoptosis. Let us consider a typical in vitro scenario where cells deprived of their native milieu experience oxidative stress concomitant with excess ROS production in mitochondria. In accordance with a negative polarity of ROS molecules their growth inevitably results in a spontaneous increase in the viscosity of the interfacial water in mitochondria. An immediate result of the increase in interfacial viscosity is a drop in the production of ATP, associated with a reduced efficiency of the mitochondrial rotary motor (ATP synthase). The reciprocity between overproduction of mitochondrial ROS and ATP synthesis is well documented in the literature. Importantly, it is possible to intervene in this relationship with light. Irradiation of cells with biostimulatory levels of NIR light (6) counteracts the destructive effect of ROS by reducing the viscosity of the interfacial water in mitochondria. As a result, the mitochondrial nano-turbine can rotate easier. For the cell this means normalization of its ATP production, as suggested by the results of recent laboratory experiments performed on model surfaces (7,8). Indeed, there is observational evidence that exposure to NIR light delivered at biostimulatory levels stimulated proliferation in cells as different as fibroblasts (9), endothelial cells (10) and neuroblastoma cells (11). Figure 1A illustrates the irradiation procedure in a typical in vitro experiment.
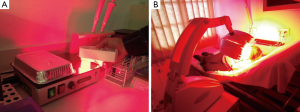
Importantly, the beneficial effect of NIR light irradiation is not restricted to cells in vitro. The same effect can be exploited for various in vivo scenarios, where cell vitality and performance are low due to inflammatory processes in association with a spontaneous increase in ROS, for instance, for the treatment of open wounds. Other conditions where irradiation with NIR light shows promising potentials include, but are not limited to, the amelioration of retinal function after light-induced photoreceptor degeneration (12) and transcranial treatment of the ischemic penumbra in stroke, or traumatic brain injuries affecting in particular military personnel (13). Despite of extensive applications in clinical practice (Figure 1B), there is still a considerable gap between current theory and practice: The predominant theoretical model, which explains the interaction of NIR light with cells, assumes that the photons are absorbed by the enzyme cytochrome c oxidase (14). In our opinion this mechanism of interaction is not satisfactory: The absorbance of cytochrome c oxidase for NIR photons is minimal—it might contribute to the synthesis of ATP but not to the expected extent. Although it is clear that more research is necessary, the experimental confirmation that interfacial water viscosity is instantly reduced by NIR light (7) puts us in the position to provide a satisfactory explanation to in vitro results and clinical data documenting the biological effect of NIR light. With the understanding of a basic mechanism progress in field is possible.
Implications for high resolution microscopy techniques
It is worth noting that the light-induced viscosity modulation is a basic physical phenomenon, therefore it is reasonable to assume the validity of similar processes for cells during extensive microscopical inspection and analysis, which will inescapably change the native state of the targeted system. Here, we consider high spatiotemporal resolution fluorescence microscopy techniques, which provide unprecedented insight into molecular processes in live cells. During their observation the cells are illuminated with extreme laser intensities reaching 1 TWm−2. Earlier we reported that laser intensities as low as 4 kWm−2 were sufficient to significantly reduce the viscosity of interfacial water (7). Remarkably, a drop in viscosity could only be measured on hydrophilic surfaces. The viscosity of these interfacial water layers, comprising 2–3 monolayers of water molecules, reaches values up to one million times higher than that of bulk water, comparable to the glue-like properties of molasses, and increases both with hydrophilicity and confinement. The elevated interfacial viscosity on hydrophilic surfaces and the possibility to reduce it with light is of considerable biological interest.
The intracellular space is known to be densely packed with cellular constituents—organelles, membranes and macromolecules—whose surfaces present a pronounced hydrophilicity, thus are masked with a glue-like hydration layer. For proteins this picture justifies the principle of the master-slave concept (15), where protein hydration layers are supposed to dictate protein folding and function. Moreover, based on the picture of a multitude of hydrophilic surfaces in a densely crowded intracellular space, it can be safely assumed that the laser light used today in many laboratories for live cell observation and analysis will interfere with both stationary states and dynamic processes in the interior of cells, for instance transcription factor residence times on DNA (16), simply by reducing interfacial water viscosity. In this context we note that 670 nm is not the only wavelength which interacts with interfacial water layers. Actually, we demonstrated that the effect is not restricted to NIR light (17). The special advantage of the wavelength 670 nm consists in its good tissue penetration without heating the fraction of bulk water in biological tissue, as opposed to infrared light.
The bottom of the Petri dish
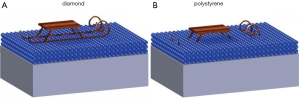
The beauty of the interfacial water viscosity model considered here consists in the fact that its validity is not restricted to the intracellular space; it is also applicable to explain the processes taking place between cells in vitro and the Petri dish. Whereas interfacial water layers masking the hydrophobic diamond surface are compact and strongly bound to the diamond surface due the hydrogen atoms connected to the nanocrystalline diamond surface, the interfacial water on the hydrophilic polystyrene is viscous and glue-like. This picture is consistent with the results of numerous experiments performed on model surfaces. In our editorial (1) we concluded that on hydrophilic polystyrene (Petri dish) the interfacial water layer acts as a scavenger for ROS molecules escaping from oxidatively stressed cells (Figure 2). Conforming to the viscous consistency of the interfacial water at the bottom of the plastic, ROS molecules were predicted to stick to the glue film, thereby experiencing an extension of their lifetime. ROS molecules accumulating in this way are likely to exert additional stress on cells in vitro. Absence of a ROS scavenger film on diamond provides a plausible explanation to the 300% gain in sperm motility observed on the diamond Petri dishes (1).
An interesting alternative model attempting to explain to the 300% gain in progressive sperm motility on diamond, compared to the continuous loss in motility on polystyrene Petri dishes, is proposed by the Batsanov group (3): “Can it be related to diamond surface having a unique property of negative electron affinity (18), thus readily releasing electrons into water?”. While the question appears to be simple, it is highly intriguing. By the special process of fabrication, a certain amount of hydrogen atoms is bound to the surface of nanocrystalline diamond. Their prevalence is involved in attracting and binding water molecules to the diamond surface. This form of diamond is a perfect electrical insulator. However, when the nanocrystalline diamond is treated with hydrogen, the resulting diamond species becomes electrically conductive (19). For this special diamond variation it is assumed (18) that electrons, which are liberated from the diamond subsurface, are taken up by a water layer on the diamond surface. Because ROS usually carry a negative electric charge, the authors of (3) suggest logically that the electrons released by the diamond surface will repel the negatively charged ROS molecules. The model ignores the aforementioned function of the interfacial water layer on the diamond surface but arrives to the same result, namely absence of ROS molecules at the substrate/cell-interface. It would not have been surprising from the evidence of recent work (18) on the conductivity of nanocrystalline diamond if the root cause of the 300% increase in progressive sperm motility was electrical repulsion between electrons and ROS molecules. But the repulsion did not take place in this way. A large number of tests showed that the culturing side of the diamond Petri dishes is practically not electrically conductive, what might be considered a very unfavorable condition for release of electrons. This is reassuring, though it does not, of course, prove that electrons were totally absent in the process under consideration. Nevertheless, the mechanism proposed by the Batsanov group is compelling. Although the validity of the model explaining the surface conductivity phenomenon on hydrogen-terminated diamond (18) was challenged by experiment (19) the release of electrons from the surface of hydrogen-terminated diamond can be regarded as ascertained. Therefore, the hypothesis put forward by the Batsanov group holds the promise for a systematic amplification of the anti-ROS quality of the diamond Petri dish. For this the diamond surface will have to be additionally terminated with hydrogen—an innovative element towards the design of the super-biocompatible Petri dish.
ROS and hydrophilicity
Destructive effects of extensive ROS bombardment are today in the focus of intensive research efforts. What has been overlooked so far is the impact of ROS on both intra- and extracellular hydrophilicity. On the nanoscale an increase in hydrophilicity accentuates the sticky nature of surfaces, thereby slowing down intracellular functions and contributing to a lifetime extension of extracellular ROS, as it is exemplarily described for the polystyrene Petri dish. Presumably, a synergistic interplay of both effects—simultaneous increase in intra- and extracellular ROS, is one of the most important elements in the factors causing biological aging. While effects of excess ROS are transient, the accompanying surface hydrophilization could induce permanent changes, especially in the extracellular matrix where an increase in hydrophilicity encourages the accumulation and durable deposition of osmiophilic groups (polar amino acids, fatty acids and calcium salts)—a progressive encapsulation process, which is believed to promote both a loss of elastic function and formation of fibrous tissue (20,21). Insight into the mechanism of formation of a variety of age related pathological conditions, including but not limited to, skin wrinkling (22), limitation of normal penis function (23) and benign prostatic hyperplasia (24), a condition where chronic prostatitis is a frequent forerunner, could help to inspire appropriate therapies, for instance, the use of NIR light. The basic principles of laser biostimulation were discovered by Professor Endre Mester (25) using the first NIR laser in history. Since then the Mester group saved more than 1,000 patients from limb amputation, most of them with ulcera cruris. Insight derived from this series of editorials invite to new research.
Acknowledgements
I thank Prof. Wolfgang Janni, Director, Department of Gynecology and Obstetrics, University Hospital Ulm, Ulm, Germany, for supporting the sperm motility project, and Dr. Frank F. Weichold, Director Critical Path and Regulatory Science Initiative, U.S. Food & Drug Administration, U.S.A. for coining the title of this letter.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Sommer AP, Jaganathan S, Maduro MR, et al. Genesis on diamonds II: contact with diamond enhances human sperm performance by 300%. Ann Transl Med 2016;4:407. [Crossref] [PubMed]
- Diamond dishes could boost IVF success rates. Available online: http://www.sciencemag.org/news/2017/04/diamond-dishes-could-boost-ivf-success-rates
- Batsanov SS, Batsanov AS. Editorial on “Genesis on diamonds II: contact with diamond enhances human sperm performance by 300% Ann Transl Med 2017;5:407. [Crossref] [PubMed]
- Lanzafame RJ. Of diamond surfaces, red light photobiomodulation and fertility: lessons from the laboratory. Ann Transl Med 2017;5:409. [Crossref] [PubMed]
- Försterling HD, Pavláth AE, Mester ÁR, et al. The sperm stewing in its own ROS—in the plastic Petri dish. Ann Transl Med 2017;5:366. [Crossref] [PubMed]
- Sommer AP, Pinheiro AL, Mester AR, et al. Biostimulatory Windows in Low-Intensity Laser Activation: Lasers, Scanners, and NASA’s Light-Emitting Diode Array System. J Clin Laser Med Surg 2001;19:29-33. [Crossref] [PubMed]
- Sommer AP, Haddad MKh, Fecht HJ. Tuning the wheel of life with light. In: Proceedings of the International Conference on Laser Applications in Life Sciences; Ulm, Germany; 2014:145.
- Sommer AP, Haddad MKh, Fecht HJ. Light Effect on Water Viscosity: Implication for ATP Biosynthesis. Sci Rep 2015;5:12029. [Crossref] [PubMed]
- Almeida-Lopes L, Rigau J, Zângaro RA, et al. Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med 2001;29:179-84. [Crossref] [PubMed]
- Schindl A, Merwald H, Schindl L, et al. Direct stimulatory effect of low-intensity 670 nm laser irradiation on human endothelial cell proliferation. Br J Dermatol 2003;148:334-6. [Crossref] [PubMed]
- Sommer AP, Bieschke J, Friedrich RP, et al. 670 nm laser light and EGCG complementarily reduce amyloid-β aggregates in human neuroblastoma cells: basis for treatment of Alzheimer's disease? Photomed Laser Surg 2012;30:54-60. [Crossref] [PubMed]
- Albarracin R, Eells J, Valter K. Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci 2011;52:3582-92. [Crossref] [PubMed]
- Miller G. Neuropathology. A battle no soldier wants to fight. Science 2011;333:517-9. [Crossref] [PubMed]
- Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B 1999;49:1-17. [Crossref] [PubMed]
- Frauenfelder H, Chen G, Berendzen J, et al. A unified model of protein dynamics. Proc Natl Acad Sci USA 2009;106:5129-34. [Crossref] [PubMed]
- Gebhardt JC, Suter DM, Roy R, et al. Single-molecule imaging of transcription factor binding to DNA in live mammalian cells. Nat. Methods 2013;10:421-6. [Crossref] [PubMed]
- Sommer AP, Zhu D, Försterling HD, et al. Crystalline water at room temperature - under water and in air. Cryst Growth Des 2008;8:2620-2. [Crossref]
- Ristein J. Surface science of diamond: familiar and amazing. Surf Sci 2006;600:3677-89. [Crossref]
- Sommer AP, Zhu D, Brühne K. Surface conductivity on hydrogen-terminated nanocrystalline diamond: Implication of ordered water layers. Cryst Growth Des 2007;7:2298-301. [Crossref]
- Stadler R, Orfanos CE. Maturation and Aging of Elastic Fibers. Arch Derm Res 1978;262:97-111. [Crossref] [PubMed]
- Fujimura T, Haketa K, Hotta M, et al. Loss of Skin Elasticity Precedes to Rapid Increase of Wrinkle Levels. J Dermatol Sci 2007;47:233-9. [Crossref] [PubMed]
- Sommer AP, Zhu D. Facial rejuvenation in the triangle of ROS. Cryst Growth Des 2009;9:4250-4. [Crossref]
- Untergasser G, Madersbacher S, Berger P. Benign prostatic hyperplasia: age-related tissue-remodeling. Exp Gerontol 2005;40:121-8. [Crossref] [PubMed]
- Ruzbarský V, Michal V. Morphologic changes in the arterial bed of the penis with aging. Relationship to the pathogenesis of impotence. Invest Urol 1977;15:194-9. [PubMed]
- Mester E, Ludány G, Sellyei M, et al. The simulating effect of low power laser rays on biological systems. Laser Rev 1968;1:3.


