Infomelanoma 2020: an online digital application designed to assist health professionals for melanoma treatment
Introduction
Melanoma is the most lethal skin tumor and its incidence has increased dramatically in the western world during the last decades. The adjusted incidence rate of melanoma in Spain is 5.2 per 100,000 inhabitants/year, with higher prevalence among women (2.7% of all female cancers) than men (1.5%). This gender imbalance is maintained across the rest of Europe (1-3). More than 5,000 new melanoma cases are diagnosed in Spain every year and up to 19–25% present with advanced stage disease, with very few options for curative treatment. Consequently, the crude mortality rate has increased from 700 to more than 900 patients per year (4-7).
New in-depth understanding of the biology of melanoma in the last 5 years has led to the identification of actionable driver mutations as well as detailed knowledge of the mechanisms that regulate the anticancer immune response. This has led to significant changes in the management of metastatic melanoma, including the approval of several BRAF and MEK inhibitors (vemurafenib, dabrafenib, trametinib and cobimetinib) as well as a variety of monoclonal antibodies targeting immune checkpoint receptors: ipilimumab (against the cytotoxic T-lymphocyte-associated protein 4, CTLA-4), nivolumab and pembrolizumab, (against the human programmed cell death 1, PD-1 receptor). The use of these new agents has modified the natural history of this disease by increasing the response rate, with an unprecedented prolongation of survival and marked improvements in quality of life (8-30) (Figure 1).
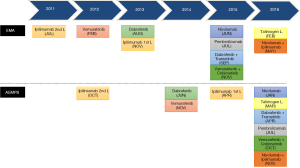
The rapidly expanding range of new therapeutic options for melanoma has also led to a significant increase in the number of clinical trials (CTs) (Figure 2) and scientific publications in this field (Figure 3), obliging healthcare professionals (HCPs) to keep constantly up to date when taking evidence-based clinical decisions. However, the sheer volume of information available, as well as the complexity in terms of interpretation and the accessibility of original platform sources can be discouraging for practicing physicians.
Herein we discuss Infomelanoma 2020 (IM2020), an online informatic tool that facilitates easy access to the most updated and relevant data on melanoma patient management, adapted to current Spanish regulations. This tool compiles and analyzes information organized into different sections: current approved treatments, CTs and centers where those trials are being conducted, and relevant key related publications. This way it can offer integrated online information that eases access to updated data on the three mentioned categories. In this paper we describe the process of development of Infomelanoma 2020 and its potential usefulness for all HCPs involved in melanoma patient care.
Material and methods
The concept, design and implementation of IM2020 were carried out by a multidisciplinary melanoma expert committee (MMEC)—including medical oncology, dermatology and pathology specialists with great experience in melanoma management, including the creation of melanoma-consensus documents and the technical support of a software engineering consultant.
The tool was developed from March 2013 to June 2016, in two sequential phases. The first was the development of Infomelanoma Gestion, a repository of: (I) key clinical and molecular characteristics that define different melanoma patient profiles; (II) the most relevant information about available treatments (ATs) and (III) active CTs and related scientific publications. The repository allows performance of searches based on key clinical and molecular characteristics of the patient, access to the most relevant information about ATs as well as CTs and related scientific publications. The second phase was the development of IM2020 online version with open access to registered melanoma specialists.
During the first stage of development, the MMEC identified and selected the most relevant clinical and molecular variables required to define the specific melanoma patient profiles, and held several meetings throughout 2013 and 2014 to reach a consensus about variables that should be addressed when establishing the search criteria for information regarding ATs, CTs and related scientific publications, as well as to test the validity of the developed algorithms.
The identification and selection of variables were based on the responses of each member of the MMEC to questions designed to elucidate the internal localization algorithm (ILA) with a deterministic approach. The three questions used were: (I) which publications of interest do you consider support the clinical variables for the choice of treatment for a patient with metastatic melanoma? (II) Which clinical variables are different in the current CTs from those that have been performed so far? (III) Taking into account all the areas involved, draw an algorithm of predictive variables you deem to be the most appropriate to guide clinicians in the best management of the patient with metastatic melanoma, and list them in order of relevance.
Regarding the first question on relevant bibliography to define these variables, the members of the MMEC agreed that several published guidelines of disease management, in all their versions (National Comprehensive Cancer Network, European Society for Medical Oncology or European Association of Dermato-oncology guidelines), clarified in a unique way the clinical variables to be taken into account in the approach to the patient with melanoma (31-33). Localization, subtypes of melanoma (cutaneous, mucous, uveal, etc.), testing of specific molecular targets (BRAF, NRAS, PDL1-2, SPARC, etc.), previous treatments, sequence of treatments and presence of brain metastases were discussed with regard to CTs.
The following clinical criteria were defined by consensus for segmenting data by the tool according to ATs, CTs and key related scientific publications: disease stage, primary melanoma site, tumor molecular characteristics (to define melanoma subtypes based on the status of key genes involved), performance status, previous treatment and presence or absence of brain metastases.
CTs selection was based on the identification of those trials currently active and recruiting in Spain. Active treatments were included based on: (I) the summary of product characteristics (SmPC) of all those drugs that had received approval for melanoma before 2011, regardless of which regulatory agency had issued its resolution; (II) from 2011, the tool would include and update datasheets of drugs approved by the European Medicines Agency (EMA).
Finally, for the category of publications, those that provided the most relevant data leading to the regulatory approval of drugs for the indication of melanoma were selected.
A careful review of all the information resources to be included in Infomelanoma Gestion was also performed during the MMEC meetings.
Results
A detailed description of the development process of the Infomelanoma Gestion and the IM2020 online version is presented below. The tool’s start-up test metrics are also shown.
Infomelanoma Gestion
For the development of Infomelanoma Gestion, the most important information on CTs, ATs and melanoma related publications—obtained from public databases including ClinicalTrials.gov (www.clinicaltrial.gov), EMA (www.ema.europe.eu) and PubMed.gov (www.ncbi.nlm.nih.gov/pubmed)—was loaded into the repository. This information was labeled piece by piece, according to the clinical variables through an ILA developed based on the following characteristics and nomenclature:
- Variables. Ordinal dichotomous or categorical items, identified by the MMEC, that may present different levels or categories and determine the elements displayed following a search profile;
- Profile or search profile. A specific combination of values of the aforementioned categories in the variables selected by user HCPs. The profile determines the outcoming elements and is a number formed with as many digits as variable values. Each profile digit corresponds to a variable and generates a single panel. Each variable modifies a digit in the profile and each digit takes the value 1, 2, 3 or 4 depending on the category of the selected variable;
- Locator. An array of digits or alphanumeric characters associated with an element linked, in turn, to the corresponding variables that indicates whether this element appears in the panel for a particular profile;
- Elements. It is estimated that the application contains ~220 items that correspond to 100 CTs, 100 scientific publications and 20 drugs. Within each element, the following relevant information is found:
- CTs: the information provided is the title, status of enrollment, experimental drugs, phase, sponsor and additional information regarding eligibility criteria and sites involved in the trial;
- Approved therapies: the tool provides trade or generic names, pharmaceutical company and the main indication according to the EMA;
- Scientific publications: the information provided includes the title, author names, date of publication, journal title and scientific level of evidence according to CONSORT criteria.
Each element has a direct link to public websites where it is possible to enrich the search accessing to more information such as SmPC—located in the EMA website—abstracts published in Pubmed.gov and clinical studies reported in Clinicaltrials.gov.
Validation of all the elements and locators assigned according to the clinical variables was performed by the MMEC.
IM2020 online version
IM2020 online version allows the elements that best fit the introduced patient profile to be found. Within the IM2020 online version, the Infomelanoma Selector tool allows the user the option to introduce all the known variables, leaving blank those that were not considered unknown. When the search tab is clicked, the system lists the results categorized as CTs, ATs and scientific publications. The user can browse the list, access all the information collected from each section and consult the original source of the element through an external direct link. Furthermore, the tool offers the possibility to generate and print all the selected information in PDF format. IM2020 can be found online on the Spanish Melanoma Group (GEM) webpage (http://www.groupgem.org/infomelanoma-2020/) and its access is restricted to the group members.
To maximize usage of the online tool, spread awareness among oncologists and facilitate the use of IM2020 from any mobile device, the flexible and scalable technology used has subsequently been extended to iOS and Android operating systems (June 2016) allowing its use in various models of smartphones and tablets. The mobile application (APP) possesses a validation and registration system for HCPs together with pre-registration for GEM members. IM2020 preserves anonymity of patients and HCPs uploading the data in compliance with Personal Data Protection Regulations in Spain (34). The APP can be downloaded through Apple App Store and Google Play Store and is connected in real time with the Infomelanoma database. Additionally, the use of the digital tools is monitored to obtain real time metrics showing qualitative and quantitative data.
IM2020 online version has currently been running since April 2015 and is directed at the GEM scientific community membership.
Start-up test metrics
The metrics of IM2020 online version have been conducted on a monthly basis from May 2015 to December 2016, and statistics of web visits, requests, most frequent patient profile prospected (Table 1), number of listed CTs, publications and ATs included in the final results report (Table 2) obtained. The results of the latest analysis found 417 accessions from May 2015 to December 2016 (Figure 4A). Regarding the APP, 46 IOS and 10 Android downloads and 32 registrations have been recorded (Figure 4B). A total of 356 searches were performed from March 2015 to December 2016 (Figure 4B).
Full table

Full table
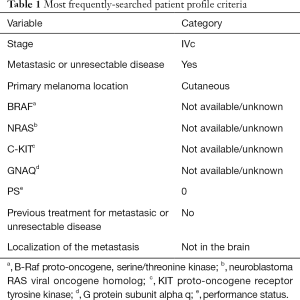
Lastly, it is worth mentioning that, so far, the majority of the IM2020 searches encompassed melanoma patients with stage IVc, with no previous treatment, BRAF mutated and with no presence of brain metastasis (Table 1).
Discussion
The amount and relevance of medical data in the field of melanoma has been steadily increasing in recent years. These fast paced advances have led to a growing need for effective compilation and accessibility of information to HCPs in order for them to take the best informed clinical decisions. In the era of Big Data, new technologies to assist in data collection, retrieval and interpretation improve clinical decision-making, increase productivity and reduce unnecessary procedures, saving time and effort, as well as diminishing the costs of care (35-39).
Here we describe the development of IM2020, a free-to-use online tool that facilitates access to relevant and updated scientific information on melanoma for Spanish-speaking oncologists and other qualified medical practitioners. The tool was designed within a framework that considers all the relevant clinical profiles needed for melanoma patient care, including prevention, early detection, tumor genomics, staging, targeted therapies, immunotherapy and CTs. Ultimately, the main objective of this tool is to improve care, facilitate data sharing and aid decision with a first-in-class tool providing a simple, intuitive and robust user experience that allows physicians to obtain in “one shot” rapid, reliable and high-quality evidence-based data regarding therapeutic recommendations, CTs and relevant key related publications.
The early metrics offered by the tool reflect pervasive use and the reliance on it. Interestingly, the number of uses has shown an increasing trend, registering a peak on May 2016 (concurring with the launch of the mobile app) followed by a decline during the following months (probably corresponding to the summer holiday period), whereas reported searches have fluctuated, reaching their highest value in June 2016.
In terms of patient profile requests, these metrics provide additional insights about the most relevant areas of interest for melanoma specialists, which can allow recognition of the unmet medical needs of certain types of patients and lead to a more customized continuous medical education and communication strategy. A robust communication plan and the development of accessible and easy-to-use tools, such as the IM2020 mobile app, offers great potential to maximize the use of the tool among oncologists and other melanoma professionals as well as rotating residents in melanoma departments of healthcare centers in Spanish-speaking countries.
It is important to note that, although we believe that IM2020 may become relevant to improve the recruitment of patients in CTs in Spain and increase knowledge of melanoma management, we acknowledge that, currently, the scope of this tool must be considered as an exploratory trial action aimed at raising awareness and generating interest in the field. However, there is much room for improvement.
A close follow-up of IM2020 use, and of input and suggestions from users, is scheduled for the coming months. Metrics will be obtained periodically and confidentially supervised and reviewed within the GEM as the most accurate way to track the real value and the perceived quality of the tool. There is no planned perspective of using or sharing the obtained data on patterns of searches, or patient profiles consulted, outside of the closed environment and objectives of the GEM, which are to improve knowledge, quality care and top class clinical and translational research for the Spanish-speaking community. Additionally, the above mentioned metrics will not have any epidemiological value, but could be offer insights into the educational needs of GEM associates or a way to facilitate more efficient CT referrals.
To fulfill its objectives, Infomelanoma Gestion database will be enriched by the MMEC according to the most updated data of the three categories—CTs (every 3 months), peer reviewed publications (every month) and ATs available in Spain (as soon as medical centers inform the GEM office)—following the same criteria established at its inception in 2014.
As a result of a better understanding of the molecular and immunological pathways associated with disease progression, future versions of the tool are expected to include not only molecular but also immunological biomarkers that are expected to change the field tailoring approved treatment to specific biological and clinical profiles (40,41).
In summary, the final value of this digital tool resides in its practicality as an aid in clinical decision-making by HCPs in their day to day activities on melanoma care. If this objective is realized, the IM2020 experience could represent a pioneering step, indicating that this digital approach can be expanded to other tumor types through a pan-tumor application or even used as a valuable resource exportable to other diseases.
Acknowledgements
We would like to acknowledge and thank Alejandro Pedrodomingo from Bio-estadística.com for providing biostatistical advisory services and GEM associates as well as its administrative office for their valuable participation and assistance with the project. Finally, we want to thank Bristol-Myers Squibb (BMS) Spain for its financial support of the Infomelanoma tool development and to express our deep appreciation of Publicis Health for their writing services.
Footnote
Conflicts of Interest: M Carrasco-Calvo is a student at Universidad San Pablo CEU and is working in the BMS Medical Department. A Berrocal received travel grant funding for conference attendance and an income for serving on the Advisory board from BMS, Roche, MSD and Novatis. A Arance received an honorarium from BMS, Roche, MSD and Novartis for serving as a speaker and on the Advisory board. S Puig receives income from Almirall, Avene, BMS, Cantabria, ISDIN, Leo, Roche and La Roche Posay for serving as a speaker and on the Advisory Board. S Martin-Algarra receives funds for lectures and also for serving on the Advisory board from BMS, Roche, MSD, and Novatis. The rest of the authors declare no conflicts of interest.
References
- Incidencia. AECC contra el cáncer 2012. Available online: http://www.aecc.es/SobreElCancer/CancerPorLocalizacion/melanoma/Paginas/incidencia.aspx
- Grupo Español Multidisciplinar de Melanoma 2017. Qué es el melanoma. Available online: http://www.groupgem.org
- Sociedad Española de Oncología Médica 2017. Epidemiología del Melanoma. Available online: http://www.seom.org/es/info-sobre-el-cancer/melanoma?start=1#content
- Lin AY, Wang PF, Li H, et al. Multicohort model for prevalence estimation of advanced malignant melanoma in the USA: an increasing public health concern. Melanoma Res 2012;22:454-9. [Crossref] [PubMed]
- Chirlaque MD, Salmerón D, Ardanaz E, et al. Cancer survival in Spain: estimate for nine major cancers. Ann Oncol 2010;21 Suppl 3:iii21-29. [Crossref] [PubMed]
- Ríos L, Nagore E, López JL, et al. Registro nacional de melanoma cutáneo. Características del tumor en el momento del diagnóstico: 15 años de experiencia. Actas Dermo-Sifiliográficas 2013;104:789-99. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France. International Agency for Research on Cancer 2013. Available online: http://globocan.iarc.fr
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [Crossref] [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [Crossref] [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [Crossref] [PubMed]
- Schadendorf D, Hodi FS, Robert C, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015;33:1889-94. [Crossref] [PubMed]
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [Crossref] [PubMed]
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. [Crossref] [PubMed]
- Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867-76. [Crossref] [PubMed]
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877-88. [Crossref] [PubMed]
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [Crossref] [PubMed]
- Wolchok JD, Hodi FS, Weber JS, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci 2013;1291:1-13. [Crossref] [PubMed]
- European Medicines Agency. London, UK; 2017. SmPC OPDIVO Nivolumab EMA. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003985/WC500189765.pdf
- European Medicines Agency. SmPC KEYTRUDA Pembrolizumab EMA. Available online: http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/003820/WC500190990.pdf
- European Medicines Agency. SmPC COTELLIC Cobimetinib EMA. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003960/WC500198563.pdf
- European Medicines Agency. SmPC YERVOY Ipilimumab EMA. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002213/WC500109299.pdf
- European Medicines Agency. SmPC TAFINLAR Dabrafenib EMA. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002604/WC500149671.pdf
- European Medicines Agency. SmPC ZELBORAF Vemurafenib EMA. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002409/WC500124317.pdf
- European Medicines Agency. SmPC MEKINIST Trametinib EMA. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002643/WC500169657.pdf
- European Medicines Agency. SmPC IMLYGIC T VEC EMA. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002771/WC500201079.pdf
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Hodi FS, Postow MA, Chesney JA, et al. Clinical response, progression-free survival (PFS), and safety in patients (pts) with advanced melanoma (MEL) receiving nivolumab (NIVO) combined with ipilimumab (IPI) vs IPI monotherapy in CheckMate 069 study. J Clin Oncol 2015;33:9004.
- Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016;17:1558-68. [Crossref] [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [Crossref] [PubMed]
- Weber JS, Minor D, D’Angelo SP, et al. A phase 3 randomized, open-label study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) versus investigator’s choice chemotherapy (ICC) in patients with advanced melanoma with prior anti-CTLA-4 therapy. The European Society for Medical Oncology 2014 Congress. Madrid, Spain. September 26-30, 2014.
- Dummer R, Hauschild A, Lindenblatt N, et al. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v126-32. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Fort Washington, US; 2017. NCCN Guidelines. NCCN Melanoma-v1 2017. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- European Association of Dermato Oncology 2016. European Guidelines. Available online: http://www.eado.org/european-guidelines/20
- History. Agencia Española de Protección de datos 2014. Available online: https://www.agpd.es/portalwebAGPD/LaAgencia/informacion_institucional/conoce/historia-iden-idphp.php
- Aungst TD. Medical applications for pharmacists using mobile devices. Ann Pharmacother 2013;47:1088-95. [Crossref] [PubMed]
- Divall P, Camosso-Stefinovic J, Baker R. The use of personal digital assistants in clinical decision making by health care professionals: a systematic review. Health Informatics J 2013;19:16-28. [Crossref] [PubMed]
- Murfin M. Know your apps: an evidence-based approach to evaluation of mobile clinical applications. J Physician Assist Educ 2013;24:38-40. [Crossref] [PubMed]
- Mickan S, Tilson JK, Atherton H, et al. Evidence of effectiveness of health care professionals using handheld computers: a scoping review of systematic reviews. J Med Internet Res 2013;15:e212. [Crossref] [PubMed]
- Mosa AS, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Med Inform Decis Mak 2012;12:67. [Crossref] [PubMed]
- Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016;16:275-87. [Crossref] [PubMed]
- Foth M, Wouters J, de Chaumont C, et al. Prognostic and predictive biomarkers in melanoma: an update. Expert Rev Mol Diagn 2016;16:223-37. [Crossref] [PubMed]



