The combination of checkpoint immunotherapy and targeted therapy in cancer
Introduction
Targeted therapies inhibit tumor growth and progression by blocking mutant proteins and signaling pathways that are essential for cell survival. With the BCR-ABL kinase inhibitor, imatinib, being the first to induce complete responses in patients with chronic myelogenous leukemia (1), now with several small molecule inhibitors of other kinases such as epidermal growth factor receptor (EGFR), BRAF, KIT, HER2 and anaplastic lymphoma kinase (ALK), selected patients can experience impressive tumor responses (2). Historically, immunotherapy has been used for the treatment of advanced stage melanoma but with small clinical benefit and substantial toxicity (3). Recently, modern immunotherapy approaches have focused on bolstering T cell responses and augmenting cell mediated immunity with final tumor destruction (4,5). This approach, known as immune checkpoint blockade (ICB), was initiated with the development of antibodies against cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) (ipilimumab and tremelimumab) and continued with the rapid clinical development of antibodies against the programmed death-1 (PD-1) T cell co-receptor (mainly nivolumab and pembrolizumab) and its ligand, B7-H1/PD-L1 (durvalumab, atezolizumab, avelumab and others). At least in the case of melanoma, it is now well known that approximately 30% of patients maintain the disease under control for many years with ICB as ipilimumab (6), nivolumab (7) or pembrolizumab (8). Similar seems to be the situation for metastatic non-small cell lung cancer (NSCLC) and triple negative breast cancer.
Due to the emergence of drug resistance clones, the responses to targeted therapies are commonly short-lived and the overall clinical benefit limited. On the other hand, the majority of cancer patients do not benefit from ICB (primary resistance) and others relapse after an initial response (acquired resistance). Adaptive resistance can also occur when the tumor is recognized by the immune system but it can adapt to the immune attack and therefore protect itself, a phenomenon that clinically manifests as primary resistance, mixed responses or acquired resistance (9). From the clinical point of view, the combination of targeted agents with immunotherapies is of interest, considering that immunotherapy can transform the important tumor responses achieved with small molecule inhibitors to durable and long-lasting remissions. Herein we will review the existing preclinical and clinical evidence on the combination of targeted therapies with ICB, mainly in melanoma and NSCLC.
The rationale and the clinical evidence of the combination of ICB with BRAF and MEK inhibitors in melanoma patients
Melanomas can escape immune destruction with an adaptive immune resistance mechanism (10). Tumor-infiltrating lymphocytes (TILs) cause their own inhibition through secretion of cytokines, like the inflammatory cytokine IFN-γ, that trigger melanocyte PD-L1 expression (10). There is strong clinical rationale behind combining BRAF inhibitor-based therapy with immunotherapy. This stems from the fact that BRAF mutant melanoma patients derive benefit from targeted therapies at least in the short term (11-15) while immunotherapy provides long-lasting responses in almost one third of the patients (6-8). There is evidence that targeted therapies that affect the mitogen-activated protein kinase (MAPK) pathway have a positive effect on immune recognition. The MAPK pathway when activated downregulates the expression of microphthalmia-associated transcription factor (MITF) (16), which ultimately leads to suppression of melanocyte-lineage antigen expression [such as gp100, melan-A, tyrosinase related proteins 1 and 2 (17,18)] that are recognized by T-cells (Figure 1). It has been shown that BRAF inhibition has favorable effects in the tumor microenvironment, including increased HLA and antigen expression, increased T-cell infiltrate, improved T-cell function, reduced immunosuppressive cytokines and increased PD-L1 expression (18-22). TILs are increased early after the initiation of BRAF inhibitor therapy (18,21). However the role of these lymphocytes is not yet fully known with some studies reporting markers of T-cell activation and others of T-cell exhaustion (23).
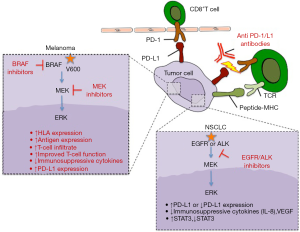
There was an initial concern that MEK inhibitors may not be as useful as BRAF inhibitors for combinations with ICB. This concern was raised from the fact that in co-cultures of autologous melanoma cells and T-cells from mice treated ex vivo with BRAF and MEK inhibitors, BRAF inhibition induced the production of INFγ and stimulated T-cell proliferation, but MEK inhibition impaired these effects (24). However, from serial tumor biopsy specimens of melanoma patients receiving BRAF and MEK inhibitors as well as from in vivo experiments with MEK inhibitors, it became clear that MEK inhibitors stimulate MITF and melanocyte-lineage antigen expression and augment T cell infiltration (18,24). Furthermore, MEK inhibitors have an additional antitumor immunity effect, by inhibiting the interaction between tumor cells and “M2-like” macrophages, which does not allow the entry of effector T-cells into the tumor (25,26). These effects make very appealing the combination of MEK inhibitors with ICB even for BRAF wild-type melanoma patients.
There are many clinical trials that are ongoing and are testing the combination of ICB (specifically anti-PD-1/L1) with BRAF and/or MEK inhibitors (Table 1). Initial reported data indicate that the triple combination is well tolerated and the efficacy is similar to what obtained with BRAF and MEK inhibition. The good tolerance does not seem to be the case for the combination of BRAF and MEK inhibitors with anti-CTLA-4 antibodies (27,28). We have reported the case of a metastatic BRAF mutant melanoma patient who achieved a pathological complete response with the sequential treatment of a BRAF and MEK inhibitor followed by CTLA-4 inhibition, but died to fatal gastrointestinal toxicity (29).
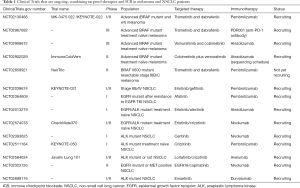
Full table
In a phase I clinical trial (ClinicalTrials.gov number NCT02027961), durvalumab was combined with dabrafenib and trametinib or with trametinib alone in BRAF/MEK inhibitor-naïve advanced melanoma patients (30) (Table 2). In both dose escalation and dose expansion phases of the study, BRAF mutant melanoma patients were treated with the triple combination (cohort A1, durvalumab at 3 mg/kg; cohort A2, durvalumab 10 mg/kg) and BRAF wild-type patients were treated with concomitant trametinib plus 10 mg/kg durvalumab (cohort B) or sequential trametinib followed by 10 mg/kg durvalumab (cohort C). The objective response rate was 100% in cohort A1, 67% in cohort A2, 21% in cohort B and 50% in cohort C (Table 2). The most frequent drug-related adverse events with the triple combination were pyrexia (63%) and fatigue (54%) and were considered manageable (30). The combination of atezolizumab, vemurafenib and cobimetinib is being tested in treatment naïve BRAF mutant advanced melanoma patients in a phase Ib study (ClinicalTrials.gov number NCT01656642). The triple combination is administered after a 28-day run-in period with vemurafenib and cobimetinib alone. The objective response rate was 93% with partial responses in 12 patients and one patient with complete response (Table 2). All adverse events observed were manageable and reversible (31). Finally, the preliminary results of the KEYNOTE 022 (ClinicalTrials.gov number NCT02130466) have been presented (32). BRAF/MEK inhibitor-naïve BRAF mutant advanced melanoma patients are included in this ongoing phase I/II study and are receiving the triple combination of pembrolizumab with dabrafenib and trametinib. The unconfirmed objective response rate in 15 evaluable patients is 60%. Overall 67% of the patients experienced grade 3–4 adverse events (Table 2) (32). In the phase II part of the trial the efficacy and safety of the triple combination is being evaluated as first line therapy for BRAF mutant melanoma patients (Table 1)
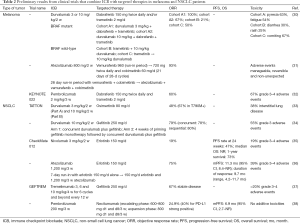
Full table
Until we have final results from the ongoing studies, the combination of ICB with targeted therapies in melanoma remains investigational. An important question that remains unanswered is the optimal sequencing of ICB and targeted therapies. We know that the positive effect of the MAPK inhibition on immune response can be found early in the course of the therapy and it disappears at the time of disease progression (21,23), but we do not know what is the status of the tumor microenvironment in patients who remain in response. The preclinical data support a positive interaction between them and the combination seems very appealing especially for BRAF mutant patients with high disease burden. However toxicity or even undermined efficacy in long term can diminish the excitement for this potential future therapeutic approach.
Is the combination of ICB with targeted therapies in NSCLC as attractive as in melanoma patients?
Oncogenic signaling processes like EGFR mutations (39) or EML4-ALK rearrangements (40) cause constitutive PD-L1 expression, but it is still unclear whether this is related with increased or decreased likelihood of responding to anti-PD-1/L1 therapies. It can be related with lack of response to other immunotherapies, since signaling through the MAPK results in the production of proteins, like VEGF, IL-8 and others, that suppress T cell recruitment and function (41) (Figure 1). The majority of EGFR mutant and ALK rearranged NSCLCs lack concurrent PD-L1 expression and high levels of CD8+ TILs, suggestive of primary immune resistance (42). EGFR mutant tumors through activating the PD-1/L1 pathway and upregulating immunosuppressive cytokines, suppress T-cell function (39). This effect can be even stronger in tumors that have undergone epithelial to mesenchymal transition (EMT) and therefore lose the expression of the immunoproteasome, which generates peptides suitable for binding onto HLA I molecules and facilitates antigen presentation for CD8+ T-cell responses (43). STAT1 is a key positive regulator of immunoproteasome subunits whereas STAT3 activation, induced by oncogenic signals (44) or EMT, has an opposing role to STAT1 inhibiting its antitumor effects and immunosurveillance (43). PD-L1 expression is significantly higher in ALK rearranged NSCLCs compared to NSCLCs with EGFR or KRAS mutation or to those with no genetic alteration of ALK, EGFR or KRAS (triple negative) (45,46). Interestingly, EGFR and ALK tyrosine kinase inhibitors (TKIs) directly inhibit tumor cell viability and indirectly enhance antitumor immunity through the PD-L1 downregulation (40,47,48). PD-L1 expression is related with better response to EGFR and ALK TKIs (45,49,50). In preclinical models, no synergistic tumor cell killing effect was observed when EGFR TKIs were combined with anti-PD-1 therapies (47). Furthermore at the time of resistance to EGFR TKIs, the PD-L1 expression changes, depending on the mechanism of resistance. For instance, EGFR TKI resistant cells with E-cadherin downregulation, one of the hallmarks of EMT, have decreased expression of PD-L1 (51).
All the above-mentioned biological knowledge and preclinical evidence indicates that the benefit from ICB in NSCLC patients with EGFR mutations or EML4-ALK rearrangements is doubtful. Indeed the subgroup analyses from ICB clinical trials have shown no benefit from anti-PD-1/L1 antibodies in NSCLC patients with EGFR mutations (52,53). In a retrospective analysis, the objective response to anti-PD-1/L1 antibodies was 3.6% for EGFR mutant and ALK rearranged NSCLC patients compared to 23.3% for EGFR and ALK wild type or those with unknown status (42). Despite these findings and as in the case of melanoma, there are ongoing clinical trials that are testing the combination of ICB with EGFR or ALK inhibitors in NSCLC patients (Table 1) and for some of them preliminary results have been reported (Table 2).
The TATTON (ClinicalTrials.gov number NCT02143466) is a multiphase Ib trial in which the 3rd generation EGFR TKI osimertinib is combined with durvalumab as well as other targeted therapies (33). Both EGFR TKI pretreated (Part A) and EGFR TKI naïve (Part B) patients were included in the study. For EGFR TKI pretreated patients, the objective response rate was 67% in the T790M positive and 21% in the T790M negative cases. The objective response rate was 67% in EGFR TKI naïve patients. These results were not that different from those obtained with osimertinib alone (54) while 38% of the patients in both parts of the study suffered interstitial lung disease (Table 2). Based on this, further enrollment of this arm of the TATTON study has been permanently suspended (33). In addition a phase III trial (ClinicalTrials.gov number NCT02454933) evaluating the combination of durvalumab with osimertinib compared to osimertinib alone in patients with T790M positive NSCLC following a prior EGFR TKI has been also suspended. Similarly to the TATTON study, another phase I open-label multicenter study (ClinicalTrials.gov number NCT02088112) was initiated to evaluate durvalumab in combination with gefitinib as first line therapy in EGFR mutant NSCLC and the expansion phase data have been reported (34). No clinically relevant differences in the objective response rate (79%) compared to what is already known for gefitinib monotherapy (73.7%) (55) in this setting were observed (Table 2). Overall 55% of the patients suffered grade 3 adverse events and treatment discontinuation due to adverse events was reported in 20% of the patients (34).
Although the results from these two early phase studies demonstrated unexpectedly high incidence of adverse events with the combination of EGFR TKIs and ICB, preliminary results from other early studies have shown promising efficacy and manageable toxicity. Specifically, in the multi-arm phase I study of nivolumab [CheckMate 012; (ClinicalTrials.gov number NCT01454102)], 21 EGFR mutant NSCLC patients (20 pretreated with erlotinib and one EGFR TKI naïve) were treated with the combination of nivolumab and erlotinib (35). The combination was associated with an acceptable toxicity profile. The objective response rate was 19% with 3 out of the 20 EGFR TKI pretreated patients and the one EGFR TKI naïve patient achieving partial response. The progression-free survival (PFS) rate at 24 weeks was 47% (Table 2). These results suggested that the combination of erlotinib plus nivolumab has an acceptable safety profile and can provide clinical benefit in EGFR mutant NSCLC patients who have developed resistance to previous EGFR TKI therapy (35). Preliminary results of the combination of erlotinib plus atezolizumab from the ongoing phase Ib study (ClinicalTrials.gov number NCT02013219) that evaluates the safety and pharmacology of atezolizumab administered with erlotinib or alectinib in patients with advanced NSCLC are available (36). The study consists of a safety-evaluation stage in patients with NSCLC regardless of EGFR status followed by an expansion stage in TKI-naïve EGFR mutant NSCLC patients (36). The authors report durable clinical responses in some patients with the combination and a manageable safety profile (36) (Table 2). In the phase I Geftrem study (ClinicalTrials.gov number NCT02040064), the safety and efficacy of the combination of tremelimumab with gefitinib is explored in EGFR mutant NSCLC patients progressing to previous EGFR TKIs (37). Disease stabilization was obtained in 67% of the patients and the toxicity profile was consistent with the previously defined adverse event profile (37) (Table 2). Finally, the combination of necitumumab and pembrolizumab in nonsquamous NSCLC patients who have progressed to platinum based chemotherapy is evaluated in a single arm multicenter phase Ib study (ClinicalTrials.gov number NCT02451930). The preliminary data indicate activity of the combination and no additive toxicity (38) (Table 2).
Other type of tumors
The lessons learned from melanoma and NSCLC are applied to other tumors. Robust synergistic responses were obtained in a prostate cancer mouse model with the combination of ICB and the multikinase inhibitor cabozantinib or the phosphoinositide 3-kinase (PI3K)/mTOR dual inhibitor BEZ235 (56). Both cabozantinib and BEZ235 diminish MDSCs and enhance ICB through inhibition of PI3K signaling and downregulation of cytokines responsible for immunosuppression-related gene expression (56). In renal cell carcinoma (RCC), antiangiogenic agents are evaluated in combination with ICB. The combination of bevacizumab and ipilimumab has already been tested in melanoma (57). Liver and other toxicities have been important issues in a phase I study combining nivolumab with sunitinib or pazopanib in patients with metastatic RCC (58) and for these reasons the ongoing studies are exploring the optimal sequencing of these agents in order to optimize the benefit of these combinations.
Conclusions
There are several issues to be addressed in the future for the combination of targeted therapies with ICB. A treatment schedule that merits to be investigated and has potential to minimize the risk of toxicities is the intermittent dosing of the targeted agent that provides enough treatment-free interval for immunotherapy administration but still is able to potentiate antigen expression and T-cell infiltration. At present combination of targeted therapies and immunotherapies for any type of tumor should be considered investigational and cannot be endorsed in the routine clinical practice.
Acknowledgements
Funding: This work was funded by La Caixa Foundation and Red Tematica de Investigacion Cooperativa en Cancer (RTICC; grant RD12/0036/ 0072).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003;348:994-1004. [Crossref] [PubMed]
- Haber DA, Gray NS, Baselga J. The evolving war on cancer. Cell 2011;145:19-24. [Crossref] [PubMed]
- Coit DG, Andtbacka R, Anker CJ, et al. Melanoma. J Natl Compr Canc Netw 2012;10:366-400. [Crossref] [PubMed]
- Luke JJ, Flaherty KT, Ribas A, et al. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14:463-82. [Crossref] [PubMed]
- Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012;12:237-51. [Crossref] [PubMed]
- Schadendorf D, Hodi FS, Robert C, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015;33:1889-94. [Crossref] [PubMed]
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [Crossref] [PubMed]
- Schachter J, Ribas A, Long G, et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival analysis of KEYNOTE-006. J Clin Oncol 2016;34:9504.
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra37. [Crossref] [PubMed]
- Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 2010;467:596-9. [Crossref] [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [Crossref] [PubMed]
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014;15:323-32. [Crossref] [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [Crossref] [PubMed]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107-14. [Crossref] [PubMed]
- Jäger E, Ringhoffer M, Karbach J, et al. Inverse relationship of melanocyte differentiation antigen expression in melanoma tissues and CD8+ cytotoxic-T-cell responses: evidence for immunoselection of antigen-loss variants in vivo. Int J Cancer 1996;66:470-6. [Crossref] [PubMed]
- Johannessen CM, Johnson LA, Piccioni F, et al. A melanocyte lineage program confers resistance to MAP kinase pathway inhibition. Nature 2013;504:138-42. [Crossref] [PubMed]
- Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013;19:1225-31. [Crossref] [PubMed]
- Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 2010;70:5213-9. [Crossref] [PubMed]
- Bradley SD, Chen Z, Melendez B, et al. BRAFV600E Co-opts a Conserved MHC Class I Internalization Pathway to Diminish Antigen Presentation and CD8+ T-cell Recognition of Melanoma. Cancer Immunol Res 2015;3:602-9. [Crossref] [PubMed]
- Wilmott JS, Long GV, Howle JR, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 2012;18:1386-94. [Crossref] [PubMed]
- Comin-Anduix B, Chodon T, Sazegar H, et al. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin Cancer Res 2010;16:6040-8. [Crossref] [PubMed]
- Cooper ZA, Reuben A, Spencer CN, et al. Distinct clinical patterns and immune infiltrates are observed at time of progression on targeted therapy versus immune checkpoint blockade for melanoma. Oncoimmunology 2016;5:e1136044. [Crossref] [PubMed]
- Hu-Lieskovan S, Mok S, Homet Moreno B, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med 2015;7:279ra41. [Crossref] [PubMed]
- Wang T, Xiao M, Ge Y, et al. BRAF Inhibition Stimulates Melanoma-Associated Macrophages to Drive Tumor Growth. Clin Cancer Res 2015;21:1652-64. [Crossref] [PubMed]
- Ebert PJ, Cheung J, Yang Y, et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016;44:609-21. [Crossref] [PubMed]
- Minor DR, Puzanov I, Callahan MK, et al. Severe gastrointestinal toxicity with administration of trametinib in combination with dabrafenib and ipilimumab. Pigment Cell Melanoma Res 2015;28:611-2. [Crossref] [PubMed]
- Ribas A, Hodi FS, Callahan M, et al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 2013;368:1365-6. [Crossref] [PubMed]
- Gonzalez-Cao M, Boada A, Teixido C, et al. Fatal gastrointestinal toxicity with ipilimumab after BRAF/MEK inhibitor combination in a melanoma patient achieving pathological complete response. Oncotarget 2016;7:56619-27. [Crossref] [PubMed]
- Ribas A, Butler M, Lutzky J, et al. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J Clin Oncol 2015;33:3003.
- Hwu P, Hamid O, Gonzalez R, et al. Preliminary safety and clinical activity of atezolizumab combined with cobimetinib and vemurafenib in BRAF V600-mutant metastatic melanoma. Ann Oncol 2016;27:1109PD.
- Hershman DL, Hodi FS, Lawrence DP, et al. Pembrolizumab (pembro) in combination with dabrafenib (D) and trametinib (T) for BRAF-mutant advanced melanoma: Phase 1 KEYNOTE-022 study. J Clin Oncol 2016;34:3014. [Crossref] [PubMed]
- Ahn MJ, Yang J, Yu H, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol 2016;11:S115. [Crossref] [PubMed]
- Gibbons DL, Chow LQ, Kim DW, et al. 57O Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): A phase I expansion in TKI-naive patients (pts) with EGFR mutant NSCLC. J Thorac Oncol 2016;11:S79. [Crossref] [PubMed]
- Gettinger S, Chow LQ, Borghaei H, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. Int J Radiat Oncol Biol Phys 2014;90:S34-5.
- Rudin C, Cervantes A, Dowlati A, et al. P3.02c-046 Safety, Clinical Activity and Biomarker Results from a Phase Ib Study of Erlotinib plus Atezolizumab in Advanced NSCLC. J Thorac Oncol 2016;12:S1302-3. [Crossref]
- Planchard D, Barlesi F, Gomez-Roca C, et al. Phase I, safety, tolerability and preliminary efficacy study of tremelimumab (Trem) in combination with gefitinib (Gef) in EGFR-mutant (EGFR-mut) NSCLC (GEFTREM). Ann Oncol 2016;27:1245P. [Crossref]
- Besse B, Garrido P, Puente J, et al. MA09.11 Efficacy and Safety of Necitumumab and Pembrolizumab Combination Therapy in Stage IV Nonsquamous Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol 2016;12:S397. [Crossref]
- Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63. [Crossref] [PubMed]
- Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 Expression by the EML4-ALK Oncoprotein and Downstream Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:4014-21. [Crossref] [PubMed]
- Liu C, Peng W, Xu C, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res 2013;19:393-403. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Tripathi SC, Peters HL, Taguchi A, et al. Immunoproteasome deficiency is a feature of non-small cell lung cancer with a mesenchymal phenotype and is associated with a poor outcome. Proc Natl Acad Sci U S A 2016;113:E1555-64. [Crossref] [PubMed]
- Chaib I, Karachaliou N, Pilotto S, et al. Co-activation of STAT3 and YES-Associated Protein 1 (YAP1) Pathway in EGFR-Mutant NSCLC. J Natl Cancer Inst 2017;109. [Crossref] [PubMed]
- Koh J, Jang JY, Keam B, et al. EML4-ALK enhances programmed cell death-ligand 1 expression in pulmonary adenocarcinoma via hypoxia-inducible factor (HIF)-1alpha and STAT3. Oncoimmunology 2015;5:e1108514. [Crossref] [PubMed]
- Busch SE, Hanke ML, Kargl J, et al. Lung Cancer Subtypes Generate Unique Immune Responses. J Immunol 2016;197:4493-503. [Crossref] [PubMed]
- Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol 2015;10:910-23. [Crossref] [PubMed]
- Hong S, Chen N, Fang W, et al. Upregulation of PD-L1 by EML4-ALK fusion protein mediates the immune escape in ALK positive NSCLC: Implication for optional anti-PD-1/PD-L1 immune therapy for ALK-TKIs sensitive and resistant NSCLC patients. Oncoimmunology 2015;5:e1094598. [Crossref] [PubMed]
- D'Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 2015;112:95-102. [Crossref] [PubMed]
- Rosell R, Palmero R. PD-L1 expression associated with better response to EGFR tyrosine kinase inhibitors. Cancer Biol Med 2015;12:71-3. [PubMed]
- Suda K, Rozeboom L, Rivard C, et al. MA15.11 Acquired Resistance Mechanisms to EGFR Kinase Inhibitors Alter PD-L1 Expression Status in Lung Cancer. J Thorac Oncol 2017;12:S433-4. [Crossref]
- Lee CK, Man J, Lord S, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 2017;12:403-7. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Lu X, Horner JW, Paul E, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 2017;543:728-32. [Crossref] [PubMed]
- Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2014;2:632-42. [Crossref] [PubMed]
- Amin A, Plimack ER, Infante JR, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2014;32:5010.


