PD-L1 as a biomarker in NSCLC: challenges and future directions
Introduction
The Food and Drug Administration (FDA) approval of immune checkpoint inhibitors has dramatically changed treatment paradigms for patients with advanced-stage or metastatic non-small cell lung cancer (NSCLC). Despite significant improvements in survival, the majority of NSCLC patients fail to respond to checkpoint inhibitors, notably antibodies targeting programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1), and uncertainties remain regarding how best to use these therapies in clinical practice. Given the risk of immune-related and other adverse effects associated with treatment, there is a need to identify biomarkers to predict which patients will and will not benefit.
Checkpoint inhibitors block inhibitory T-cell signaling, thereby leading to an endogenous antitumor immune response. PD-1 is a transmembrane immunoregulatory molecule responsible for the negative regulation of T cell activation and peripheral tolerance. It is expressed on T cells, B cells, and natural killer (NK) cells and binds to its ligands PD-L1 and PD-L2 (1). Expression of PD-L1 rarely occurs on normal tissues but is prevalent on antigen presenting cells (APCs) and tumor cells in numerous solid malignancies including NSCLC. There is constitutive expression of PD-L1 on tumor cells, which occurs during oncogenic processes in a state of chronic antigen presentation, and inducible expression of PD-L1 at the tumor site in the presence of pro-inflammatory cytokines such as interferon-gamma.
Over the past several years, studies have demonstrated improved outcomes with checkpoint inhibitors compared to conventional chemotherapy in advanced NSCLC (2-5). The expression of PD-L1 on tumor cell membranes via immunohistochemistry (IHC) has been most widely studied for use with anti-PD-1/PD-L1 therapy in this setting. While its role as a companion or complementary diagnostic assay in the refractory setting has been studied extensively, an even more important role for PD-L1 has emerged for selecting patients for upfront treatment. With numerous trials focusing on first-line immunotherapy, patient selection is more important than ever. As the field continues to advance, we must keep in mind that there are some limitations to using PD-L1 as a predictive biomarker in NSCLC, and this commentary will focus on some of those issues.
Predictive value of PD-L1 testing in NSCLC
PD-L1 in previously treated NSCLC patients
Among patients with previously treated NSCLC, anti-PD-1/PD-L1 agents have consistently demonstrated improvements in overall survival (OS) compared to chemotherapy (Table 1). The phase III CheckMate (CM) 017 and 057 studies evaluated the role of nivolumab 3 mg/kg every 2 weeks in previously treated squamous and non-squamous advanced NSCLC, respectively (2,6). Both studies demonstrated statistically significant improvements in OS: 9.2 months for nivolumab compared to 6.0 months for docetaxel in CM 017 (HR 0.59, P<0.001) and 12.2 months for nivolumab compared to 9.4 months for docetaxel in CM 057 (HR 0.73, P=0.002). PD-L1 expression was assessed using the Dako 28-8 assay, and there was no minimum PD-L1 expression level required for study entry or primary endpoint analysis.

Full table
Retrospective evaluation of pre-treatment biopsies was conducted for PD-L1 expression at three pre-specified cutoff points: 1%, 5%, and 10%. In CM 017 (squamous NSCLC), PD-L1 expression was neither prognostic nor predictive of benefit to nivolumab. However, CM 057 (non-squamous NSCLC) did show a predictive association between PD-L1 expression and OS benefit with nivolumab compared to chemotherapy: P=0.06 at 1% PD-L1 expression, P<0.001 for 5% and 10% expression levels.
Intriguingly, in an analysis of early survival for patients in CM 057, Peters and colleagues showed that patients with poor prognostic factors (<3 months since last treatment, progressive disease as best response to prior treatment, ECOG performance status of 1), in conjunction with low or no PD-L1 expression, were at a higher risk of death within the first 3 months of treatment with nivolumab compared to docetaxel, partly explaining the non-proportional hazards seen in this study (OS curves crossed ~7 months) (13). However, most patients treated with nivolumab with low or no PD-L1 expression did not die within the first 3 months and many had durable treatment benefit with nivolumab, a finding that suggests PD-L1 score alone is insufficient for patient selection for nivolumab. An important takeaway from this subgroup analysis is that perhaps a combination of factors, rather than PD-L1 expression alone, is needed to help inform treatment decisions.
In KEYNOTE (KN)-010, advanced, previously treated NSCLC patients with a tumor proportion score (TPS) of at least 1%, as measured by the Dako 22C3 assay, were enrolled to receive pembrolizumab (either 2 or 10 mg/kg every 3 weeks) or docetaxel. Both the pembrolizumab 2 and 10 mg/kg arms had improvements in OS compared to docetaxel: 10.4 months with pembrolizumab 2 mg/kg (HR 0.71, P=0.008), 12.7 months with pembrolizumab 10 mg/kg (HR 0.61, P<0.0001), and 8.5 months with docetaxel (4). While the study was enriched for tumors expressing PD-L1, it was evident that higher expression levels were predictive of improved survival benefit—the HR for death for tumors with TPS ≥50% was 0.53 compared to 0.76 for TPS 1–49%.
The phase III OAK study compared atezolizumab 1,200 mg every 3 weeks to standard therapy with docetaxel in previously treated squamous and non-squamous NSCLC (9). PD-L1 expression was measured using the Ventana SP142 assay but no minimum cut-off level was required for study entry. OS was 13.8 months in the atezolizumab compared to 9.6 months in the docetaxel arm (HR 0.73, P=0.0003). Subgroup analysis demonstrated OS improvements regardless of PD-L1 expression levels, though it was noted that tumors expressing high levels of PD-L1 on tumor cells (≥50% or TC3) or tumor infiltrating immune cells (≥10% or IC3) derived the most benefit from atezolizumab treatment with a HR for death of 0.41 (95% CI, 0.27–0.64).
The phase III ARCTIC study evaluating durvalumab in advanced NSCLC patients who have received at least two prior systemic chemotherapy regimens is ongoing, and preliminary results are not yet reported (11).
PD-L1 in treatment-naïve NSCLC patients
Immune checkpoint blockade, as a single-agent or in combination with cytotoxic chemotherapy, has recently moved into the first-line setting. Pembrolizumab was FDA-approved for up-front treatment in October 2016 for patients with ≥50% PD-L1 expression based on results from the phase III KN-024 trial (8). In this study, treatment-naïve stage IV NSCLC patients with ≥50% PD-L1 staining were randomized 1:1 to pembrolizumab 200 mg versus four to six cycles of platinum-doublet chemotherapy with a primary endpoint of PFS. PD-L1 expression was assessed using the Dako 22C3 assay. Of the 1,653 patients screened with evaluable tumor tissue, 30% were found to have tumors with ≥50% PD-L1 staining. Crossover from the chemotherapy group to the pembrolizumab group was permitted in the event of disease progression. The ORR was significantly improved with pembrolizumab (45% in the pembrolizumab arm vs. 28% in the chemotherapy arm). PFS was improved by 4.3 months in the pembrolizumab group (median PFS 10.3 vs. 6.0 months, HR 0.50, P<0.001) and most markedly for those patients with squamous histology (HR 0.35). Most importantly, OS was significantly longer in the pembrolizumab group (HR 0.60, 95% CI, 0.41–0.89, P=0.005).
More recently, the FDA granted accelerated approval to pembrolizumab in combination with platinum-based chemotherapy in first-line NSCLC (non-squamous histology), irrespective of PD-L1 expression, based on results from the KN-021 trial (14). This phase II study, which capitalizes on the immunological effects of chemotherapy to improve the efficacy of immunotherapy, demonstrated significant improvements in ORR (55% vs. 29%) and PFS (HR 0.53, P=0.01) for patients treated with combination therapy (pembrolizumab with carboplatin and pemetrexed) compared to chemotherapy alone. In the pembrolizumab plus chemotherapy arm, the proportion of patients who achieved an objective response was similar in patients with PD-L1 TPS <1% and those with a score of ≥1% (57% vs. 54%, respectively). There was a suggestion of higher proportion of responses in patients with a TPS of ≥50% (5 of 19 patients with TPS 1–49% vs. 16 of 20 patients with TPS ≥50%), but sample sizes were too small for significance.
In comparison to the KN-024 trial, the phase III CM 026 trial evaluating the efficacy of first-line nivolumab in stage IV/recurrent NSCLC with PD-L1 positive tumors (defined as staining in ≥1% of tumors cells using the Dako 28-8 antibody) demonstrated no PFS (4.2 months with nivolumab vs. 5.9 months with chemotherapy, HR 1.15, 95% CI, 0.91–1.45, P=0.25) or OS (14.4 vs. 13.2 months, HR 1.02; 95% CI, 0.80–1.30) benefit in patients with a PD-L1 expression level of ≥5% (7). Interestingly, the PFS curves separated early (chemotherapy arm performing better than nivolumab) but then later converged about 7 months post randomization, perhaps pointing to the late and/or sustained effects of immunotherapy.
Why did KN-024 meet its primary endpoint when CM 026 did not? The difference is unlikely due to biological differences between nivolumab and pembrolizumab, especially given the similarities seen in large trials of previously treated patients (e.g., CM 017, 057, and KN-010). The most obvious reason is the difference in PD-L1 cutoff, although this cannot explain the entire story, as a subgroup analysis from CM 026 suggested that even patients whose tumors harbored PD-L1 levels of at least 50% did not demonstrate a significantly improved PFS or OS benefit with nivolumab compared to chemotherapy. Other factors may include differences in trial design (prior radiation, glucocorticoid use during trial) and baseline patient characteristics (smoking history, sex). Perhaps most importantly, the above trials highlight the imprecise relationship between PD-L1 expression and therapeutic benefit to anti-PD-1/PD-L1 therapy, as well as fundamental differences in the PD-L1 assays. Specifically, there is no data to suggest a threshold PD-L1 expression level of 50% using Dako 28-8 equates to a similar cutoff using Dako 22C3 assay. As such, given that pembrolizumab improves outcomes in NSCLC patients with a TPS ≥50% using Dako 22C3, it is quite possible that nivolumab, and furthermore atezolizumab and durvalumab, may also demonstrate survival benefits in the first-line setting if the correct assay cutoff is applied to the correct patient population.
First line, phase III trials involving durvalumab and atezolizumab are ongoing (Table 1) (10,12).
Challenges with PD-L1 as a biomarker in NSCLC
Assay-specific challenges
The availability of four PD-L1 diagnostic assays, each individualized for a specific anti-PD-1/PD-L1 agent (Table 1), poses a daunting challenge for patients, clinicians, and other stakeholders seeking access to treatment without overly burdensome diagnostic costs and procedures. Each of these antibody clones are raised against different epitopes on the PD-L1 molecule: Ventana SP142 and SP263 target the intracellular domain on PD-L1, while Dako 22C3 and 28-8 clones are raised against epitopes within the extracellular domain (15). Moreover, there are separate immunostaining protocols that depend on different antigen retrieval conditions and staining platforms. Recognizing these hurdles, a novel industry-academic collaboration, the Blueprint PD-L1 Assay Comparison Project, was developed by the FDA, American Association for Cancer Research (AACR), American Society of Clinical Oncology (ASCO), as well as four pharmaceutical companies (Bristol-Myers Squibb, Merck & Co. Inc., AstraZeneca PLC, and Genentech, Inc.), and two diagnostic companies (Agilent Technologies, Inc./Dako Corp and Roche/Ventana Medical Systems, Inc.) to bring clarity to this topic.
The Phase I portion of the project included 38 formalin-fixed, paraffin-embedded (FFPE) NSCLC samples that were selected for analysis by expert pathologists trained in interpretation of the individual assays (2 pathologists from Ventana and 1 from Dako) (16). Overall 19 of the 38 cases (50%) demonstrated concordant positive staining above the cutoffs utilized by the four assays, while 14 (37%) cases showed discordance in positive staining, and 5 (13%) cases showed negative staining regardless of the assay used. Further analysis revealed three of the assays (28-8, 22C3, and SP263) had similar performance with respect to staining of tumor cells. However, the fourth assay (Ventana SP142), consistently labeled fewer tumor cells (Figure 1).
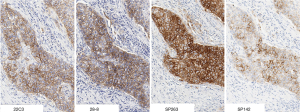
In contrast to the other assays that focus on PD-L1 expression on tumor cells, SP142 is designed to detect PD-L1 expression on both tumor cells (TC) and tumor-infiltrating immune cells (IC), based on the assumption that quantifying expression on both cell types might better predict response than either alone. In clinical trials such as OAK, threshold levels are defined as TC1 or IC1 for PD-L1 expression on 1% or more of TC or IC, TC2 or IC2 for PD-L1 expression on 5% of these cells, TC3 as PD-L1 expression on 50% or more of TC, and IC3 as PD-L1 expression on 10% or more of IC. However, evaluating immune cells for PD-L1 staining is a procedure that is less standardized and less prevalent in clinical practice. As a result, the Blueprint paper found there was decreased concordance among pathologists when looking at PD-L1 expression on immune cells, regardless of the assay used. Both of these findings—decreased PD-L1 staining by SP142 and increased discrepancy for immune cell staining—have been confirmed in a separate multi-institutional study (17). From a practical standpoint, given that atezolizumab is approved in the second-line setting without any PD-L1 restrictions, it is unclear what benefit the SP142 assay adds in the routine management of advanced NSCLC.
Beyond SP142, the lack of standardization highlighted by Blueprint has real implications for clinical care. In this paper, over a third of cases had discordant PD-L1 expression results. As a result, had treatment decisions been based on these results—for example, determining upfront treatment with pembrolizumab for patients with PD-L1 expression >50%—a substantial number of patients would not have received treatment if an alternative assay (and corresponding cut-off value) was used.
PD-L1 expression on tumor cells and the tumor microenvironment (TME) exists on a spectrum and can vary dramatically due to a number of factors (described in further detail below). However, all currently available assays and phase III trials use specific cutoff values (Table 1) to define positivity as a binary result. This is problematic for the individual assays, which can act as both biomarker and gatekeeper for a specific drug, but even more so when making inter-assay comparisons. As seen in the Blueprint study, while very high and no PD-L1 expression were for the most part concordant among assays, low to moderate expression levels that are seen in the majority of NSCLC patients can result in discrepancy. In such situations, rather than using a specific cutoff, a continuous measure may better approximate treatment efficacy.
While the preliminary phase I Blueprint results are limited in their ability to inform clinical decision making, the phase II portion of this collaborative initiative is ongoing and will hopefully provide more clarity for clinical practice. For now, the available data suggest the role of immune cell staining is limited at best and highlight the need for harmonization with regards to PD-L1 staining on tumor cells.
Biopsy-specific challenges
Outside of the technical aspects of inter-assay variability, there is growing data to suggest that specific features of a tumor specimen undergoing PD-L1 testing have a profound impact on assay results. For the most part, PD-L1 testing is performed on histologic specimens. However, recent reports suggest that cytology specimens may provide enough cellularity for some of the assays mentioned. A pilot study revealed that 92% (34 of 37 cases) of cytology specimens had sufficient cellularity for analysis with 22C3 (greater than 100 cells) (18). Although there were limited paired cytology-histology samples to guide concordance testing, using cytology for PD-L1 testing may be a feasible option particularly if a core biopsy or resection specimen is unavailable.
Furthermore, based on the findings in KN-010, PD-L1 testing on archival tissue appears to provide similar clinical information as fresh samples, which thankfully obviates the need for fresh biopsies (4). Deciding from which site (primary tumor vs. metastatic lesion) to obtain a biopsy can depend on a variety of factors including accessibility, operator preference, etc. However, when considering PD-L1 testing on a biopsy specimen, it is important to realize that levels can vary significantly depending on where the tissue was procured. In one study, discordant PD-L1 expression levels were seen in 14% of cases when paired primary lung and brain metastases were compared (19). In a separate study of 109 patients with resected stage II and III lung adenocarcinomas, conflicting PD-L1 expression levels between primary tumor and nodal metastases were seen in 38% of cases (20).
While such intertumoral heterogeneity raises questions about choosing the most appropriate site for PD-L1 testing, notable differences in expression even within a tumor are also cause for concern. Given that less than a fraction of a percent of a tumor is generally evaluated on biopsy and that the adaptive immune response is quite dynamic, some intratumoral spatial discordance may be expected. However, studies have documented marked variations in PD-L1 levels between high expression areas, such as the leading edge dividing tumor and stroma, and low expression zones (21). The use of Automated Quantitative Analysis (AQUA) may present a more effective method of characterizing heterogeneous PD-L1 expression (22). This technique, developed by Rimm and colleagues, quantifies PD-L1 expression in tumors through the use of high-resolution automated image acquisition and a set of algorithms that can distinguish tumor from stromal elements using quantitative immunofluorescence (QIF). It has been shown to correlate with both disease-free and OS in laryngeal squamous cell carcinoma (23). Whether this holds true for NSCLC remains to be seen, though such rapid, quantitative analysis of PD-L1 expression could provide more global and accurate measurements of PD-L1 levels across and within sites of disease.
However, until larger trials can evaluate which sites and areas of tumor to evaluate for PD-L1 expression or the techniques such as AQUA are validated and become more widespread, it can be challenging for clinicians to interpret biopsy results. Notably, the majority of clinical trials leading to FDA approvals for PD-1/PD-L1 inhibitors allowed for physician discretion (except with regards to cytology which was generally insufficient for eligibility) when selecting the site of biopsy for PD-L1 testing.
Patient-specific challenges
Approximately 20% of NSCLC adenocarcinomas seen in the United States have characterized molecular alterations, most notably epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) translocations, that drive cancer growth. While targeted therapies have become standard of care for these patients, anti-PD-1/PD-L1 therapies have failed to improve upon outcomes further. Initial studies including CM 057 and KN-010 demonstrated no difference in outcomes among EGFR-mutant patients treated with nivolumab or pembrolizumab when compared to docetaxel, respectively (4,6). Intriguingly, despite low response rates to these agents, both EML4-ALK rearrangements and EGFR mutations appear to upregulate PD-L1 expression in NSCLC cell lines and mouse models (24,25).
In a retrospective analysis, patients who harbored these molecular genotypes, the majority of whom (82%) had progressed on prior tyrosine kinase inhibitor therapy, and received treated with anti-PD-1/PD-L1 therapy, had low objective responses rates—3.6% for EGFR-mutant and 23% for ALK-positive patients (26). While PD-L1 levels were variable (ranging from 24–60% for PD-L1 >1% using the E1L3N assay), the presence of CD8+ tumor-infiltrating lymphocytes (TILs) along with high PD-L1 levels was rare. This “non-inflamed” TME may explain the lack of responsiveness to anti-PD-1/PD-L1 agents in these tumors. Furthermore, these mutations are often seen in non-smokers, whose tumors also have fewer non-synonymous mutations from a lack of carcinogen exposure (27). Taken together, these findings suggest that smoking status and mutational load may predict better efficacy to PD-1/PD-L1 inhibitors than PD-L1 expression levels in patients with EGFR mutations or EML4-ALK rearrangements. There are ongoing trials determining the efficacy of combination tyrosine kinase inhibitors with PD-1/PD-L1 inhibitors in this population.
Conclusions and future directions
Clearly, PD-L1 is an imperfect and dynamic biomarker with deficiencies related not only to the assays but also to the intrinsic qualities of the tumor (Table 2). However, there is little doubt that we have entered a new era in which first-line treatment for metastatic disease need no longer be a platinum-based doublet alone. With KN-024, patients with PD-L1 levels ≥50%, representing approximately 25% of NSCLC patients, should receive pembrolizumab up-front. It is in this context that PD-L1 testing, using the Dako 22C3 assay, is most useful.

Full table
For treatment-naïve, non-squamous patients with lower PD-L1 levels, the results of KN-021 provide an alternative approach to first-line chemotherapy alone, although we await OS data and phase III trials confirming these preliminary results. The Dako 22C3 PD-L1 assay may help discriminate when to use the combination vs. pembrolizumab alone in the first-line setting for patients with PD-L1 expression ≥50%. It remains to be seen whether there is an added benefit for those patients or patients with squamous histology at any PD-L1 level to receive combination anti-PD-1/PD-L1 therapy and chemotherapy given the increase in toxicity
Moving forward, a negative PD-L1 score may help decision-making with other immunotherapy combinations, such as combined anti-CTLA-4/anti-PD-1/PD-L1 blockade (28) or enrollment in a clinical trial with novel checkpoint inhibitors targeting LAG, OX40, IDO, CD137, etc. At the same time, efforts are ongoing to clarify the role of anti-PD-1/PD-L1 therapy outside of metastatic NSCLC. The small-cell lung cancer (SCLC) cohort of CM-032, evaluating combination nivolumab and ipilimumab, saw objective responses in one-third of patients treated with combination therapy – a remarkable improvement from the 7% response rate seen historically with single agent topotecan in refractory SCLC (29). Furthermore, phase III ANVIL (NCT02595944) and PEARLS (NCT02504372) trials seek to evaluate nivolumab and pembrolizumab, respectively, as adjuvant therapy for patients with resected stage IB to IIIA NSCLC. As we further expand the role of these drugs, it will become more important to refine biomarkers for patient selection. Beyond PD-L1, other areas of interest include tumor mutational burden, neoantigen signature, inflammatory immune profiling using multiplex IHC, or QIF/AQUA.
There are certainly frustrations with PD-L1, but its dynamic nature also speaks to the dynamic interaction between the immune system and tumor. As we continue to learn more about immunotherapy, we will continue to strive for that elusive cure. Already, we have made incredible progress from the days of conventional chemotherapy.
Acknowledgements
None.
Footnote
Conflicts of interest: CA Shu has received consulting fees from Genentech. The other authors have no conflicts of interest to declare.
References
- Salmaninejad A, Khoramshahi V, Azani A, et al. PD-1 and cancer: molecular mechanisms and polymorphisms. Immunogenetics 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Herbst R, De Marinis F, Jassem J, et al. editors. Phase III clinical trials of atezolizumab compared with standard chemotherapy in PD-L1–selected chemotherapy-naïve patients with advanced NSCLC. ESMO Asia 2015 Congress; 2015; Singapore.
- Planchard D, Yokoi T, McCleod MJ, et al. A Phase III Study of Durvalumab (MEDI4736) With or Without Tremelimumab for Previously Treated Patients With Advanced NSCLC: Rationale and Protocol Design of the ARCTIC Study. Clin Lung Cancer 2016;17:232-6.e1. [Crossref] [PubMed]
- Peters S, Antonia S, Goldberg SB, et al. 191TiP: MYSTIC: a global, phase 3 study of durvalumab (MEDI4736) plus tremelimumab combination therapy or durvalumab monotherapy versus platinum-based chemotherapy (CT) in the first-line treatment of patients (pts) with advanced stage IV NSCLC. J Thorac Oncol 2016;11:S139-40. [Crossref] [PubMed]
- Peters S, Cappuzzo F, Horn L, et al. OA03.05 Analysis of Early Survival in Patients with Advanced Non-Squamous NSCLC Treated with Nivolumab vs Docetaxel in CheckMate 057. J Thorac Oncol 2017;12:S253. [Crossref]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Rebelatto MC, Midha A, Mistry A, et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol 2016;11:95. [Crossref] [PubMed]
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the "Blueprint PD-L1 IHC Assay Comparison Project". J Thorac Oncol 2017;12:208-22. [Crossref] [PubMed]
- Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol 2017;3:1051-58. [Crossref] [PubMed]
- Heymann J, Pagan C, Crapanzano J, et al. editors. PD-L1 Expression in Non-Small Cell Lung Carcinoma (NSCLC): Feasibility of Cytology and Its Comparison with Resection and Small Biopsies 2017 USCAP Annual Meeting; 2017 Mar 04–10; San Antonio, TX, USA.
- Mansfield AS, Aubry MC, Moser JC, et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol 2016;27:1953-8. [Crossref] [PubMed]
- Uruga H, Bozkurtlar E, Huynh TG, et al. Programmed Cell Death Ligand (PD-L1) Expression in Stage II and III Lung Adenocarcinomas and Nodal Metastases. J Thorac Oncol 2017;12:458-66. [Crossref] [PubMed]
- McLaughlin J, Han G, Schalper KA, et al. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol 2016;2:46-54. [Crossref] [PubMed]
- Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med 2002;8:1323-7. [Crossref] [PubMed]
- Vassilakopoulou M, Avgeris M, Velcheti V, et al. Evaluation of PD-L1 Expression and Associated Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma. Clin Cancer Res 2016;22:704-13. [Crossref] [PubMed]
- Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 Expression by the EML4-ALK Oncoprotein and Downstream Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:4014-21. [Crossref] [PubMed]
- Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol 2015;10:910-23. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science 2013;339:1546-58. [Crossref] [PubMed]
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]


