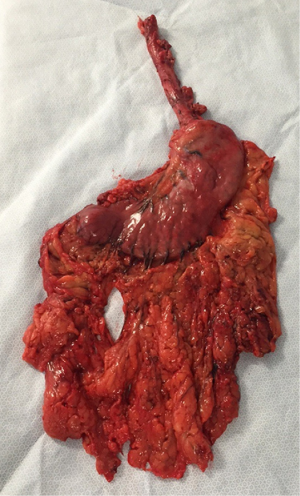
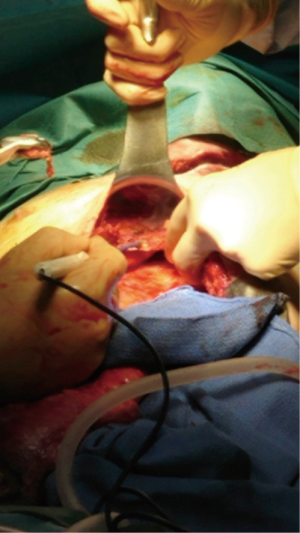
Peculiarities of intra-thoracic colon interposition—eso-coloplasty: indications, surgical management and outcomes
Introduction
Stomach is the usual organ of choice for oesophageal replacement. Gastric pull-up is a standardized, fast and secure procedure, requiring only one anastomosis either in the thorax or in the neck, and is usually performed with mini-invasive techniques (1-3).
Colon interposition was first described in 1911, and remained the organ of choice for oesophageal replacement during the first half of the 20th century (4). Nowadays, it is mainly used when the stomach is not available, for tumours of the upper oesophagus or the hypopharynx, or for benign diseases (5).
This paper will focus mainly on the indications of intra-thoracic colon inter-position, the specific early complications of this surgery and the long-term follow-up. We will also briefly describe the specific aspects of the surgical technique.
Indications
Nowadays, a colon graft is chosen as the organ of choice for oesophageal replacement instead of the stomach in very specific situations.
The stomach cannot be used during an esophagectomy for cancer
When patients have a history of gastrectomy, either for carcinomas or for peptic disease, or when the stomach must be removed at the same time (Siewert III tumors) another organ of replacement must be chosen (5-10) (Figure 1). A Colon graft is the organ of replacement of choice in this situation. Both intra-thoracic and cervical anastomoses can be performed, depending of the extent of the oesophageal disease and the resection needed.
Some teams use preferentially a jejunum graft, performing an intra-thoracic anastomosis on a Roux-en-Y loop. But when compared to a colon graft, utilisation of the jejunum is more complex, the transplant length is limited, and only experienced teams perform it. Overall, the indications of jejunal interposition are limited (Table 1) (11).

Full table
The stomach is deliberately not chosen as the organ of replacement
For tumours of the upper oesophagus or of the hypopharynx, a long length of graft is necessary (12). Thus, a long colonic transplant, with a good vascularisation, can be a better option for an organ of replacement in order to avoid the presence of ischemic tissue at the upper part of the graft. And ultimately avoid necrosis of the graft or leakage of the anastomosis.
For benign disease in young patients, such as end-stage achalasia or strictures secondary to caustic and peptic injuries, several teams report good outcomes when colon is used over the stomach as a replacement for the oesophagus (7,13,14). Because the stomach and its function as a reservoir are kept intact, it allows a good quality of life for the patients. Thus, dumping syndrome, nausea and vomiting, regurgitation, reflux and oesophagitis are less frequent, and the quality of life increases (15). Finally, the stomach is still available if the graft fails, or if secondary complications arise.
The specific case of pediatrics: atresia of the esophagus
There is no consensus on which organ of replacement must be chosen in the case of atresia of the oesophagus (16). Gastric transposition in children has shown excellent results, with more than 90% of good or excellent outcomes (17). Colon interposition offers good results as well, and is a valid second choice for the treatment of atresia with more than 50% of the patients asymptomatic in the long term.
Colon interposition is also a possible choice for treatment of strictures secondary to caustic burn injuries and complex peptic stenosis (18).
Surgical technique
The surgical technique of colon interposition has been described in detail elsewhere (10,19,20). This section will focus on the main steps of the surgery, as well as the precautions that must be taken for intra-thoracic colon grafts.
Pre-operative examinations
For patients over 45 to 50 years old, or with a history of colic surgery or abdominal aneurysm, an angiography of the colic vessels should be considered (21). The examination should also be performed for patients with atherosclerosis, with potential affected visceral arteries.
Bowel preparation should be performed during the days preceding the surgery associating cathartics and an appropriate diet, or iterative water enemas through a jejunostomy when oral feeding is not possible.
Choice and preparation of the graft
Depending of the primary disease, two types of colon transplants can be chosen: either a short one or a long one.
Short colic transplant with an intra-thoracic eso-colic anastomosis are considered for:
- Tumours of the gastro-oesophageal junction;
- Benign stenosis of the lower third of the oesophagus;
- End-stage functional disorders.
Long colic transplant with a cervical anastomosis are chosen for:
- Sub-total esophagectomy (caustic burn, papillomatosis, carcinoma of the oesophagus with a previous gastric resection);
- Oesophageal exclusion;
- Impossible use of the posterior mediastinum as the route of reconstruction.
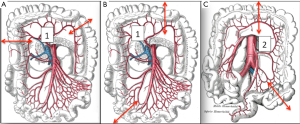
Intra-operative findings, especially regarding the vascular supply, can influence the choice of the graft (Figure 2):
- Short transverse colon: vascularised by the middle colic artery;
- Long transverse colon: vascularised by the left colonic vessels;
- Long right colon with the terminal ileum: vascularised by the middle colic artery.
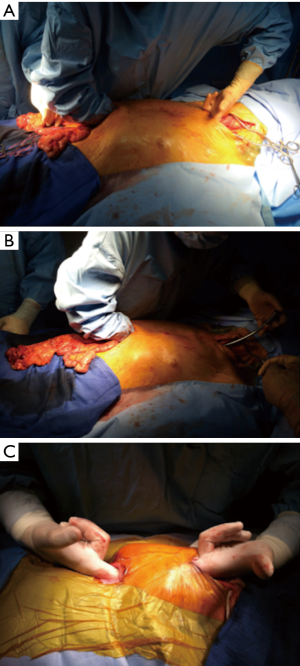
Whatever the choice made, it is mandatory to check the correct vascularisation of the graft after the mobilization of the colon, and identification of all the vascular supplies (Figure 3) (22). The Right colic transplant has the additional benefit of an ulterior rescue surgery in case of a failed transposition, with a possible second coloplasty based on the transverse colon.
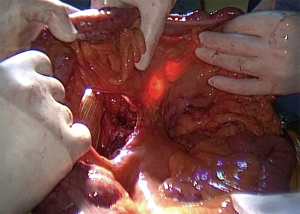
Route of reconstruction (Figure 4)

When the oesophagus is resected as the same time as the colon interposition, the transplant is usually placed through the posterior mediastinum. For short colonic transplant with an intra-thoracic eso-colic anastomosis, it is the only route available. For long colonic transplants other routes can be chosen.
When the colon interposition is performed after the esophagectomy, for example after a caustic injury with esophageal exclusion, or a failed gastric tube during a previous surgery, it is placed through a retro-sternal route. If the posterior mediastinum is hostile, previous radiotherapy or future one planned, it is also the route of choice (Figure 5).
When the retro-sternal route is not available, the graft can be placed sub-cutaneously in front of the sternum (Figure 6). Some teams choose preferentially this route of reconstruction, though the reported leakage rate is high (11).
Exceptionally, the colic graft can be place through the pleura, with an associated phrenotomy. For example when the posterior mediastinum is unavailable, and the patient has a history of sternotomy. But the results are disappointing and this route should be avoided if possible.
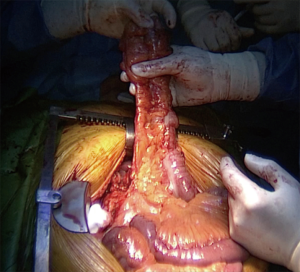
Whatever the route chosen, the length of the transplant must be checked, and the intra-thoracic graft should then be positioned as straight as possible to avoid future redundancy (Figure 7).
Surgical approaches (Table 2)
Full table
Several approaches can be described. They are dependent of two factors: firstly if the oesophagus is resected at the same time as the reconstruction, secondly if a short or a long transplant is needed.
The Oesophagus is resected at the same time as the coloplasty: the posterior mediastinum is free.
- For long colonic transplants: a right thoracic approach is performed for the dissection of the oesophagus, whether through thoracotomy or thoracoscopy. In a second time a median laparotomy is performed and a left cervicotomy for the anastomosis;
- For short colonic transplant, first a laparotomy is performed for the preparation of the colon, followed by a right thoracic approach for the dissection of the oesophagus and the intra-thoracic anastomosis;
- As an alternative to this approach, some teams prefer a left thoraco-phreno-laparotomy: the esophagectomy and the reconstruction with a short colonic transplant are performed at the same time.
The oesophagus has already been resected, the posterior mediastinum is unavailable and another route must be chosen.
- A laparotomy is performed for preparation of the graft, followed by and a left cervicotomy for eso-colic anastomosis.
Anastomoses
When the stomach is left in place, three anastomoses are performed:
- The eso-colic anastomosis, either in the neck or in the thorax (Figure 8);
- The colo-gastric anastomosis;
- And finally the colo-colic one.
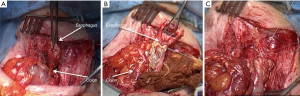
When the stomach has been removed four anastomoses are needed, because of the Roux-en-Y loop performed between the distal extremity of the colic transplant and the jejunum. Thus the colo-gastric anastomosis is replaced by a colo-jejunal anastomosis and a jejuno-jejunal one.
Additional steps, superdrainage and supercharge techniques, can be performed on the eso-colic anastomosis to improve the vascular supply of the graft (23,24).
Outcomes and complications
Because of the complexity of colon interposition, high mortality and morbidity rates are observed. Thus, the reported mortality ranges from 0 to more than 16% for some teams, and the associated risk of graft necrosis ranges from 0 to 10%, and 0 to 15% for anastomotic leaks (5-11,25-31) (Table 3). Risk factors such as diabetes, cardiovascular diseases and chronic obstructive pulmonary disease (COPD) have been described and should be addressed before the surgery (32). During the surgery, compression on the colon graft should be avoided at all cost, in order to preserve the transplant. Thus, dissection of the retro-sternal path or the thoracic inlet should be done carefully. If needed the plate of the manubrium, the clavicle or the first rib can be removed as well (33).
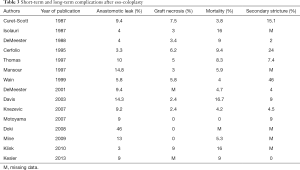
Full table
The management of failed colon transplant can be challenging, and optimal medical treatment (nutrition resuscitation, sepsis control, limitation of inflammation…) should be performed in association with surgery (33). Whenever it is possible, if removal of a necrotic transplant must be performed, as much length of the colic graft should be preserved for future reconstruction.
One of the main complications of gastric pull-up is biliary reflux and secondary reflux disease in the graft, leading to alimentary discomfort (3,34). For young patients with benign diseases, especially if a vagal sparing esophagectomy has been performed, the rate of biliary reflux and associated symptoms decreases. Thus in the long term, the overall quality of life reported after colon interposition is good (5,14,35-38). Symptoms such as diarrhea, dysphagia, reflux and dumping syndrome are observed in the early post-operative period but improve over time (10). Chronic aspirations are needed in less than 10% of the patients.
Two additional complications are described in the long term.
Stricture of the graft occurs in 0 to 40% of patients, and is more often observed in overweight patients or after anastomotic leaks. Most of the time, strictures are managed successfully with endoscopic dilatations (5,7,9,14,25,28,29,39).
The exact number of patients needing revision surgery remains unknown, whether it is in the early post-operative period for anastomotic leakage or necrosis of the transplant, or in the long-term. Redundancy of the graft is one of the causes of late revision surgery, where segmental resection of the colic transplant is usually performed. The frequency increases over time, the reported rate ranging from 0 to 40% (33,40). During the initial surgery, several precautions should be taken in order to limit the rate of redundancy: performing first the oesophago-colic anastomosis, measuring the graft carefully in order for the transplant to have a course as straight as possible, remove any structure potentially obstructing, anchoring the anti-mesenteric border to the crura or the diaphragmatic opening (5,8,20,40).
Conclusions
Colon interposition is a complex surgery with specific indications. It has a high morbidity and mortality, though in the long term the survival rate is similar to gastric pull-up and the quality of life is good. Thus it remains a valid choice for oesophageal replacement.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Akiyama H, Miyazono H, Tsurumaru M, et al. Use of the stomach as an esophageal substitute. Ann Surg 1978;188:606-10. [Crossref] [PubMed]
- Sugarbaker DJ, DeCamp MM. Selecting the surgical approach to cancer of the esophagus. Chest 1993;103:410S-4S. [Crossref] [PubMed]
- Greene CL, DeMeester SR, Worrell SG, et al. Alimentary satisfaction, gastrointestinal symptoms, and quality of life 10 or more years after esophagectomy with gastric pull-up. J Thorac Cardiovasc Surg 2014;147:909-14. [Crossref] [PubMed]
- Kelling G. Oesophagoplastic mitt Hilfe des Querkolon. Zentralbl Chir 1911;38:1209-12.
- Thomas P, Fuentes P, Giudicelli R, et al. Colon interposition for esophageal replacement: current indications and long-term function. Ann Thorac Surg 1997;64:757-64. [Crossref] [PubMed]
- Isolauri J, Markkula H, Autio V. Colon interposition in the treatment of carcinoma of the esophagus and gastric cardia. Ann Thorac Surg 1987;43:420-4. [Crossref] [PubMed]
- Curet-Scott MJ, Ferguson MK, Little AG, et al. Colon interposition for benign esophageal disease. Surgery 1987;102:568-74. [PubMed]
- DeMeester TR, Johansson KE, Franze I, et al. Indications, surgical technique, and long-term functional results of colon interposition or bypass. Ann Surg 1988;208:460-74. [Crossref] [PubMed]
- Cerfolio RJ, Allen MS, Deschamps C, et al. Esophageal replacement by colon interposition. Ann Thorac Surg 1995;59:1382-4. [Crossref] [PubMed]
- Mine S, Udagawa H, Tsutsumi K, et al. Colon interposition after esophagectomy with extended lymphadenectomy for esophageal cancer. Ann Thorac Surg 2009;88:1647-53. [Crossref] [PubMed]
- Doki Y, Okada K, Miyata H, et al. Long-term and short-term evaluation of esophageal reconstruction using the colon or the jejunum in esophageal cancer patients after gastrectomy. Dis Esophagus 2008;21:132-8. [Crossref] [PubMed]
- Morita M, Saeki H, Ito S, et al. Technical improvement of total pharyngo-laryngo-esophagectomy for esophageal cancer and head and neck cancer. Ann Surg Oncol 2014;21:1671-7. [Crossref] [PubMed]
- Kitajima T, Momose K, Lee S, et al. Benign esophageal stricture after thermal injury treated with esophagectomy and ileocolon interposition. World J Gastroenterol 2014;20:9205-9. [PubMed]
- Knezević JD, Radovanović NS, Simić AP, et al. Colon interposition in the treatment of esophageal caustic strictures: 40 years of experience. Dis Esophagus 2007;20:530-4. [Crossref] [PubMed]
- Yildirim S, Köksal H, Celayir F, et al. Colonic interposition vs. gastric pull-up after total esophagectomy. J Gastrointest Surg 2004;8:675-8. [Crossref] [PubMed]
- Rintala RJ, Sistonen S, Pakarinen MP. Outcome of esophageal atresia beyond childhood. Semin Pediatr Surg 2009;18:50-6. [Crossref] [PubMed]
- Spitz L. Gastric transposition in children. Semin Pediatr Surg 2009;18:30-3. [Crossref] [PubMed]
- Spitz L. Esophageal replacement: overcoming the need. J Pediatr Surg 2014;49:849-52. [Crossref] [PubMed]
- Thomas PA, Gilardoni A, Trousse D, et al. Colon interposition for oesophageal replacement. Multimed Man Cardiothorac Surg 2009;2009:mmcts.2007.002956.
- Gust L, Ouattara M, Coosemans W, et al. European perspective in Thoracic surgery-eso-coloplasty: when and how? J Thorac Dis 2016;8:S387-98. [Crossref] [PubMed]
- McDermott S, Deipolyi A, Walker T, et al. Role of preoperative angiography in colon interposition surgery. Diagn Interv Radiol 2012;18:314-8. [PubMed]
- Sakorafas GH, Zouros E, Peros G. Applied vascular anatomy of the colon and rectum: clinical implications for the surgical oncologist. Surg Oncol 2006;15:243-55. [Crossref] [PubMed]
- Saeki H, Morita M, Harada N, et al. Esophageal replacement by colon interposition with microvascular surgery for patients with thoracic esophageal cancer: the utility of superdrainage. Dis Esophagus 2013;26:50-6. [Crossref] [PubMed]
- Lu HI, Kuo YR, Chien CY. Extended left colon interposition for pharyngoesophageal reconstruction using distal-end arterial enhancement. Microsurgery 2008;28:424-8. [Crossref] [PubMed]
- DeMeester SR. Colon interposition following esophagectomy. Dis Esophagus 2001;14:169-72. [Crossref] [PubMed]
- Mansour KA, Bryan FC, Carlson GW. Bowel interposition for esophageal replacement: twenty-five-year experience. Ann Thorac Surg 1997;64:752-6. [Crossref] [PubMed]
- Wain JC, Wright CD, Kuo EY, et al. Long-segment colon interposition for acquired esophageal disease. Ann Thorac Surg 1999;67:313-7; discussion 317-8. [Crossref] [PubMed]
- Davis PA, Law S, Wong J. Colonic interposition after esophagectomy for cancer. Arch Surg 2003;138:303-8. [Crossref] [PubMed]
- Motoyama S, Kitamura M, Saito R, et al. Surgical outcome of colon interposition by the posterior mediastinal route for thoracic esophageal cancer. Ann Thorac Surg 2007;83:1273-8. [Crossref] [PubMed]
- Klink CD, Binnebösel M, Schneider M, et al. Operative outcome of colon interposition in the treatment of esophageal cancer: a 20-year experience. Surgery 2010;147:491-6. [Crossref] [PubMed]
- Kesler KA, Pillai ST, Birdas TJ, et al. “Supercharged” isoperistaltic colon interposition for long-segment esophageal reconstruction. Ann Thorac Surg 2013;95:1162-8; discussion 1168-9. [Crossref] [PubMed]
- Briel JW, Tamhankar AP, Hagen JA, et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg 2004;198:536-41; discussion 541-2. [Crossref] [PubMed]
- de Delva PE, Morse CR, Austen WG Jr, et al. Surgical management of failed colon interposition. Eur J Cardiothorac Surg 2008;34:432-7; discussion 437. [Crossref] [PubMed]
- D'Journo XB, Martin J, Ferraro P, et al. The esophageal remnant after gastric interposition. Dis Esophagus 2008;21:377-88. [Crossref] [PubMed]
- Burgos L, Barrena S, Andrés AM, et al. Colonic interposition for esophageal replacement in children remains a good choice: 33-year median follow-up of 65 patients. J Pediatr Surg 2010;45:341-5. [Crossref] [PubMed]
- Metzger J, Degen L, Beglinger C, et al. Clinical outcome and quality of life after gastric and distal esophagus replacement with an ileocolon interposition. J Gastrointest Surg 1999;3:383-8. [Crossref] [PubMed]
- Cense HA, Visser MR, van Sandick JW, et al. Quality of life after colon interposition by necessity for esophageal cancer replacement. J Surg Oncol 2004;88:32-8. [Crossref] [PubMed]
- Greene CL, DeMeester SR, Augustin F, et al. Long-term quality of life and alimentary satisfaction after esophagectomy with colon interposition. Ann Thorac Surg 2014;98:1713-9; discussion 1719-20.
- Jeyasingham K, Lerut T, Belsey RH. Functional and mechanical sequelae of colon interposition for benign oesophageal disease. Eur J Cardiothorac Surg 1999;15:327-31; discussion 331-2. [Crossref] [PubMed]
- Strauss DC, Forshaw MJ, Tandon RC, et al. Surgical management of colonic redundancy following esophageal replacement. Dis Esophagus 2008;21:E1-5. [Crossref] [PubMed]