Right heart function during acute respiratory distress syndrome
Historical and physiological background
Acute respiratory distress syndrome (ARDS) is still associated with high mortality (1) and long-term disability (2). This poor outcome is probably more the consequence of circulatory failure than hypoxemia per se (3). In ARDS, septic shock is responsible for circulatory failure in half of patients (4), but awareness of its management is high worldwide (5). On the other hand, for the other half of patients, hemodynamic instability is directly related to ARDS as a result of pulmonary circulation dysfunction and its consequence, which is right ventricular (RV) failure. The RV is now consensually considered as the weakest link during ARDS, and its failure, in the most severe form, is named acute cor pulmonale (ACP) (6). The RV is in all points opposite to the left ventricle. With its sensitive systolic function (7) and a tolerant diastolic function, the RV acts as a passive crescent chamber promoting venous return from the systemic circulation to the pulmonary one (8). Without any contractile reserve (9), the RV has no adaptive mechanism other than its dilatation when its afterload is increased, even a little. Any ventriculo-arterial uncoupling between the RV and the pulmonary circulation leads to RV dilatation, which is responsible for LV compression on the one hand and an ischemic vicious circle for itself on the other hand. ARDS is also a disease of RV afterload resulting in ventriculo-arterial uncoupling as a consequence of the increase in pulmonary vascular resistance (10-12). But pulmonary arterial hypertension is generated by numerous factors that can be schematically classified as “intrinsic”, resulting from alveolar and capillary injuries (12), actions on which are limited, and “extrinsic”, which are mainly the consequences of mechanical ventilation, on which the ventilator strategy has to focus (13). Severe ACP, as well as pulmonary dysfunction, has been shown to independently increase mortality during ARDS (14,15), showing that uncoupling between the RV and the pulmonary circulation is at the core of the prognosis. Here we will briefly describe the main known risk factors for ACP in ARDS and will then propose another reading of recent ARDS studies, mainly based on their potential protective effect on the RV. This will help us to propose testing of an RV protective approach as a new respiratory strategy in ARDS.
Risk factors for ACP in ARDS
Besides pneumonia as the cause of ARDS, 3 parameters have recently been demonstrated as independently associated with ACP (15): driving pressure ≥18 cmH2O, PaCO2 ≥48 cmH2O and PaO2/FiO2 ratio <150 mmHg.
Driving pressure, which is the difference between the end-inspiratory alveolar pressure and the total positive end-expiratory pressure (PEEP), reflects the pressure induced by tidal ventilation. It is, then, related to the respiratory strategy (in which tidal volume is increased) and to the severity of ARDS (how depressed the respiratory compliance is). In clinical practice, esophageal pressure is usually not measured and driving pressure is used as a surrogate of transpulmonary pressure, at least in patients without significant depression in chest wall compliance. For nearly 50 years, it has been well known that transpulmonary pressure exponentially increases pulmonary vascular resistance (16,17). High transpulmonary pressure participates in pulmonary hypertension and is responsible for cyclic alteration of RV function during tidal ventilation (18). Interestingly, it has recently been shown that a driving pressure >18 cmH2O is associated with increased mortality (19). Very recently, Villar et al. also nicely demonstrated that for a plateau pressure maintained below 30 cmH2O, survival decreased when driving pressure was equal to or higher than 19 cmH2O (20). Unfortunately, no RV evaluation was proposed in these studies, but one can assume that the impact of driving pressure on prognosis could be explained in part by its impact on the RV.
During the first twenty years of ARDS ventilatory support, one of the main objectives was to maintain carbon dioxide in the normal range. At the beginning of the 1990s, the application of a lung protective approach with permissive hypercapnia opened up a new era during which the main objective was to limit tidal volume, so as to decrease the phenomenon of volume-induced lung injury (21). The enthusiasm associated with this concept was strengthened by the description of the pleomorphic beneficial effect of carbon dioxide (22), and some authors proposed enlarging the concept of permissive hypercapnia to one of “therapeutic” hypercapnia (23). However, hypercapnia was also described as a powerful vasoconstrictor of the pulmonary circulation (24), and its deleterious effects have been more deeply understood since the beginning of the 2000s. Some authors nicely showed very recently that severe hypercapnia, defined as a PaCO2 ≥50 mmHg, is independently associated with mortality in moderate to severe ARDS (25). A few years ago, before showing that a PaCO2 ≥48 mmHg was associated with ACP in a large cohort of ARDS patients (15), we reported in a small number of patients with severe ARDS that an abrupt increase in PaCO2 applied for only one and a half hours, even associated with a decreased driving pressure, is responsible for severe ACP and hemodynamic alterations (26). Once again, putting these data together, we might understand the deleterious effect of severe hypercapnia on prognosis as the consequence of its effect on the pulmonary circulation and the RV, even though no study has directly reported such a link.
The PaO2/FiO2 ratio is routinely used to evaluate the severity of lung injury, while many confounding factors have been reported, as changes in cardiac output (27) and low PvO2 effect (28). A ratio below 150 mmHg is also associated with ACP (15). The effect of low oxygen levels on the pulmonary circulation has long been known. Enson et al. reported that the greater the oxygen unsaturation, the higher the mean pulmonary arterial pressure (29). And this is obviously true whatever the cause of such a decrease in oxygenation, i.e., severe lung injury or associated compromised hemodynamics. It is interesting to note that the only study on prone positioning (PP) that reported a significant increase in survival is the one which included patients with a PaO2/FiO2 ratio below 150 mmHg (30). In this study, PP very significantly increased survival as well as the number of days free from cardiovascular dysfunction, and decreased the number of cardiac arrests (30), suggesting that hemodynamics plays a major role in the effect of PP on survival. Indeed, PP is also well known to unload the RV (31,32) and we can reasonably assume that improvement in RV function during PP could be one of the main mechanisms explaining the amelioration of survival. PP has very few limitations and can also be proposed for obese patients (33) and after abdominal surgery (34).
How to protect the RV?
As suggested above, the RV protective approach is intended to control the three main factors associated with RV failure, so as to alleviate RV afterload and, as a consequence, decrease mortality (Figure 1). This ventilatory strategy relies on a triptych associating increase in PaO2/FiO2 >150 mmHg, decrease in driving pressure <18 cmH2O and control of PaCO2 under 48 mmHg (35). PP is the cornerstone of this strategy (36), since it increases oxygenation (without increasing PEEP) and decreases PaCO2 and driving pressure (37), which explains its beneficial effects on the RV (31).
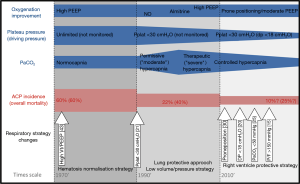
To apply an RV protective approach, daily monitoring of the RV by echocardiography is essential (38). If echocardiography is not available 24/24, experts recently proposed an algorithm for hemodynamic monitoring based on pulse pressure respiratory variations (39). Pulse pressure variations have to be considered as a signal which obliges intensivists to look for hypovolemia, but mostly for RV failure (39) confirmed by an echocardiogram simply showing RV dilatation and paradoxical septal motion (40). Detecting RV failure by daily echocardiography could help intensivists to control risk factors better and to apply PP whatever the PaO2/FiO2 ratio. Further research would help determine whether other treatments designed to correct pulmonary capillary dysfunction, such as sevoflurane (41) or cell-based therapy (42), are synergistic with PP.
Conclusions
ARDS is associated with a poor outcome, in which RV failure plays a major role. The RV appears to be the weak link and correction of the ventriculo-arterial uncoupling between the RV and the pulmonary arterial circulation could be at the core of the ventilatory strategy. The RV protective strategy, which relies on PP and RV monitoring by echocardiography, could be promising and should be further evaluated in a randomized control trial.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293-304. [Crossref] [PubMed]
- Vieillard-Baron A, Girou E, Valente E, et al. Predictors of mortality in acute respiratory distress syndrome. Focus On the role of right heart catheterization. Am J Respir Crit Care Med 2000;161:1597-601. [PubMed]
- Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 2004;30:51-61. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Repessé X, Charron C, Vieillard-Baron A. Acute respiratory distress syndrome: the heart side of the moon. Curr Opin Crit Care 2016;22:38-44. [Crossref] [PubMed]
- Redington AN, Rigby ML, Shinebourne EA, et al. Changes in the pressure-volume relation of the right ventricle when its loading conditions are modified. Br Heart J 1990;63:45-9. [Crossref] [PubMed]
- Guyton AC, Lindsey AW, Abernathy B, et al. Venous return at various right atrial pressures and the normal venous return curve. Am J Physiol 1957;189:609-15. [PubMed]
- Spruijt OA, de Man FS, Groepenhoff H, et al. The effects of exercise on right ventricular contractility and right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med 2015;191:1050-7. [Crossref] [PubMed]
- Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 2012;122:2731-40. [Crossref] [PubMed]
- Price LC, McAuley DF, Marino PS, et al. Pathophysiology of pulmonary hypertension in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2012;302:L803-15. [Crossref] [PubMed]
- Zapol WM, Kobayashi K, Snider MT, et al. Vascular obstruction causes pulmonary hypertension in severe acute respiratory failure. Chest 1977;71:306-7. [Crossref] [PubMed]
- Moloney ED, Evans TW. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur Respir J 2003;21:720-7. [Crossref] [PubMed]
- Bull TM, Clark B, McFann K, et al. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med 2010;182:1123-8. [Crossref] [PubMed]
- Mekontso Dessap A, Boissier F, Charron C, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 2016;42:862-70. [Crossref] [PubMed]
- West JB, Dollery CT, Naimark A. Distribution of Blood Flow in Isolated Lung; Relation to Vascular and Alveolar Pressures. J Appl Physiol 1964;19:713-24. [PubMed]
- Whittenberger JL, McGregor M, Berglund E, et al. Influence of state of inflation of the lung on pulmonary vascular resistance. J Appl Physiol 1960;15:878-82. [PubMed]
- Vieillard-Baron A, Loubières Y, Schmitt JM, et al. Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol 1999;87:1644-50. [PubMed]
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Villar J, Martin-Rodriguez C, Dominguez-Berrot AM, et al. A Quantile Analysis of plateau and driving pressures: effects on mortality in patients with acute respiratory distress syndrome receiving lung-protective ventilation. Crit Care Med 2017;45:843-50. [Crossref] [PubMed]
- Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med 1990;16:372-7. [Crossref] [PubMed]
- Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill--too little of a good thing? Lancet 1999;354:1283-6. [Crossref] [PubMed]
- Hickling KG, Walsh J, Henderson S, et al. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med 1994;22:1568-78. [Crossref] [PubMed]
- Viitanen A, Salmenpera M, Heinonen J. Right ventricular response to hypercarbia after cardiac surgery. Anesthesiology 1990;73:393-400. [Crossref] [PubMed]
- Nin N, Muriel A, Penuelas O, et al. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med 2017;43:200-8. [Crossref] [PubMed]
- Mekontso Dessap A, Charron C, Devaquet J, et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med 2009;35:1850-8. [Crossref] [PubMed]
- Dantzker DR, Lynch JP, Weg JG. Depression of cardiac output is a mechanism of shunt reduction in the therapy of acute respiratory failure. Chest 1980;77:636-42. [Crossref] [PubMed]
- Lemaire F, Jardin F, Regnier B, et al. Pulmonary gas exchange during venoarterial bypass with a membrane lung for acute respiratory failure. J Thorac Cardiovasc Surg 1978;75:839-46. [PubMed]
- Enson Y, Giuntini C, Lewis ML, et al. The Influence of Hydrogen Ion Concentration and Hypoxia on the Pulmonary Circulation. J Clin Invest 1964;43:1146-62. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Vieillard-Baron A, Charron C, Caille V, et al. Prone positioning unloads the right ventricle in severe ARDS. Chest 2007;132:1440-6. [Crossref] [PubMed]
- Jozwiak M, Teboul JL, Anguel N, et al. Beneficial hemodynamic effects of prone positioning in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2013;188:1428-33. [Crossref] [PubMed]
- De Jong A, Molinari N, Sebbane M, et al. Feasibility and effectiveness of prone position in morbidly obese patients with ARDS: a case-control clinical study. Chest 2013;143:1554-61. [Crossref] [PubMed]
- Gaudry S, Tuffet S, Lukaszewicz AC, et al. Prone positioning in acute respiratory distress syndrome after abdominal surgery: a multicenter retrospective study: SAPRONADONF (Study of Ards and PRONe position After abDOmiNal surgery in France). Ann Intensive Care 2017;7:21-9. [Crossref] [PubMed]
- Repessé X, Charron C, Vieillard-Baron A. Acute cor pulmonale in ARDS: rationale for protecting the right ventricle. Chest 2015;147:259-65. [Crossref] [PubMed]
- Vieillard-Baron A, Price LC, Matthay MA. Acute cor pulmonale in ARDS. Intensive Care Med 2013;39:1836-8. [Crossref] [PubMed]
- Vieillard-Baron A, Rabiller A, Chergui K, et al. Prone position improves mechanics and alveolar ventilation in acute respiratory distress syndrome. Intensive Care Med 2005;31:220-6. [Crossref] [PubMed]
- Vieillard-Baron A, Prin S, Chergui K, et al. Echo-Doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am J Respir Crit Care Med 2002;166:1310-9. [Crossref] [PubMed]
- Vieillard-Baron A, Matthay M, Teboul JL, et al. Experts' opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med 2016;42:739-49. [Crossref] [PubMed]
- Jardin F, Dubourg O, Bourdarias JP. Echocardiographic pattern of acute cor pulmonale. Chest 1997;111:209-17. [Crossref] [PubMed]
- Jabaudon M, Boucher P, Imhoff E, et al. Sevoflurane for Sedation in Acute Respiratory Distress Syndrome. A Randomized Controlled Pilot Study. Am J Respir Crit Care Med 2017;195:792-800. [Crossref] [PubMed]
- Laffey JG, Matthay MA. Fifty Years of Research in ARDS. Cell Based Therapy for ARDS: Biology and Potential Therapeutic Value. Am J Respir Crit Care Med 2017. [Epub ahead of print]. [Crossref] [PubMed]


