Synovial sarcoma of the chest wall: a case report and literature review
Abstract: Synovial sarcoma is a malignant soft-tissue tumor that most commonly occurs in the extremities of young adults. Synovial sarcoma arising from the chest wall is rare and only some cases had been reported in the literature. We present a 57-year-old woman who presented with chest pain. Radiologic evaluation revealed a right parietal tumor destructing the mid-portion of the 8th rib, with heterogeneous enhancement and invasion of the pectoral muscle and extra pleural fat. A surgical resection consisting in parietectomy was achieved. The histological and immunohistochemical findings were consistent with synovial sarcoma. An adjuvant chemotherapy was prescribed but the patient was lost of view. She presented 6 months later with a recurrent huge parietal mass.
Keywords: Synovial sarcoma; chest wall; surgery; histology
Introduction
Synovial sarcoma is a rare soft tissue tumor with a high grade of malignancy developing mostly near large joints. The most common sites of origin are the thigh, knee, ankle, foot, and upper extremities. Primary synovial sarcoma of the chest wall is rare.
Observation
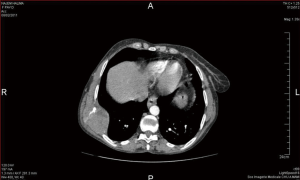
A 57-year-old woman was explored for chest pain for 6 months. On physical examination, a somewhat nodular, non movable, tender subcutaneous mass was noted on the right anterior chest wall, overlying the 7th and 8th ribs. Chest X-ray showed a large mass involving the lower portion of the right chest with partial bony destruction of 6th and 7th ribs. Chest computed tomography showed a large, heterogeneous, enhanced mass in the right hemithorax centered on the body of the 8th rib measuring 6.9 cm × 4.4 cm × 5.7 cm. It invades the serratus anterior muscle and the extra-pleural fat, sparing the lung inside (Figure 1). Computed tomography of the brain and the abdomen-pelvis revealed no pathological findings. A fine needle biopsy was achieved and was histopathologically consistent with high grade sarcoma.
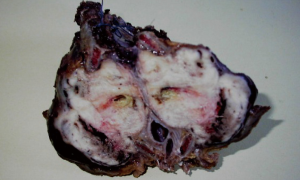
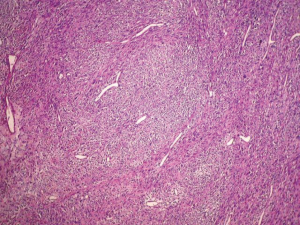
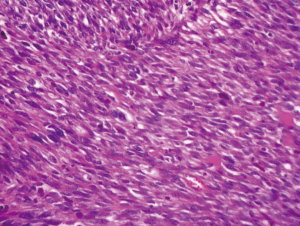
The patient was planned for parietectomy and the tumor was removed with involved ribs. Macroscopically, the mass measuring, 12 cm × 5.5 cm × 3 cm was firm, relatively well-circumscribed, lobulated with a gray white cut surface. Hemorrhage, necrotic and cystic changes were noted. It invaded widely the 8th rib and the adjacent soft tissue of 6th and 7th ribs (Figure 2). Pathologically, the tumor showed a malignant spindle cell proliferation poorly differentiated alternating cellular zones with less cellular areas displaying myxoid and micro cystic changes. Tumor cells were arranged in nodules, sheets and long fascicles with sometimes hemangiopericytoid growth pattern (Figures 3,4). They were large, relatively uniform with quit undefined cell borders. Nuclei were elongated or ovoid, vesicular and markedly atypical. The mitotic rate is up to 22 mitoses per 10 fields on HP. Necrosis was estimated to less than 50% of the tumor surface. The tumor invaded the 8th rib and the adjacent soft tissue of 6th and 7th ribs. Resection margins were negative. On immunohistochemistry, the tumor cells were positive for vimentin, CD99, Bcl2 and S 100 protein. They were actin, desmin, CD34, cytokeratin and EMA negative. These findings were consistent with synovial sarcoma of chest wall.
The postoperative course was uneventful. An adjuvant chemotherapy with adriamycin and Holoxan was prescribed but the patient was lost of view. She presented 6 months later with a recurrent huge parietal mass. Computed tomography showed a recurrent parietal mass with lung controlateral metastasis. The patient died three days after.
Comments
Synovial sarcomas are rare malignant neoplasms of unknown histogenesis, most commonly affecting the lower extremities and frequently arises adjacent to joints or tendon sheaths.
Synovial sarcoma is a misnomer because the tumor does not arise from the synovium; it only resembles synovial tissue at light microscopy. Since it appears to arise from as yet unknown multipotent stem cells that are capable of differentiating into mesenchymal and/or epithelial structures and lack synovial differentiation (1).
Synovial sarcomas has been found to account for approximately 5-14% of soft tissue sarcomas in different studies (2,3).
A part from the extremities, synovial sarcoma may arise within head and neck, esophagus, retroperitoneum and also in the thorax; mediastinum, heart, lung, pleura or pericardium with lesser frequency (4,5).
Synovial sarcomas rarely involve the chest wall. Less than 10 cases were reported in the literature (6-15). There is a mild male predominance, the sex ratio is 1.5. It affects preferentially young patients (16). The symptoms of synovial sarcoma of the chest wall depend on the structures undergoing compression or invasion from the tumor. Patients may present with chest pain, cough, dyspnea, reduced breath sounds and weight loss (10).
At computed tomography, synovial sarcoma that arises in the chest wall is characterized most commonly as a heterogeneously enhanced mass with well-defined margins, cortical bone destruction, tumor calcifications and tumor infiltration of the chest wall musculature (12,17). Mediastinal, hilar, diaphragmatic or axillary lymph nodes are rarely involved (12).
Macroscopically, tumors are round or multilobular, poorly or well circumscribed. They may be either unencapsulated or surrounded by a thin fibrous capsule (18). On section, they were gray-yellow and exhibited a rather variegated appearance with cyst formation, hemorrhage, and necrosis. Gross calcification may be evident (19).
Synovial sarcoma is composed of two morphologically different types of cells: epithelial cells, resembling those of carcinoma, and fibrosarcoma-like spindle cells. Depending on the relative prominence of the two cellular elements and the degree of differentiation, synovial sarcomas form a continuous morphologic spectrum and can be broadly classified into the (I) biphasic type, with distinct epithelial and spindle cell components in varying proportions; (II) monophasic fibrous type; (III) rare monophasic epithelial type and (IV) poorly differentiated (round cell) type (20). Almost all morphological subtypes are characterized by a specific t(X;18) (p11.2; q11.2) chromosomal translocation.
Like other soft tissue sarcomas, synovial sarcoma’s diagnosis is difficult to establish purely on the basis of histological appearance. It is even difficult in some cases without an obvious biphasic differentiation. Thence, immunohistochemical studies must be completed showing neoplastic cells diffusely immunoreactive to CK, EMA, Vimentin, Bcl-2, Actin and CD99 with focal immunoreactivity for S-100 protein and are negative for CD34 and Desmin (21).
The monophasic fibrous synovial sarcoma (such our case) may resemble a number of other spindle cell neoplasms including fibrosarcoma, leiomyosarcoma, MPNST, hemangiopericytoma, and spindle cell carcinoma. In fact, the spindle cells of fibrosarcoma appear in a binds of interlaced arrangement, mitotic figures are common, and epithelial markers are negative. The spindle cells of leiomyosarcoma have a dark eosinophilic cytoplasm and Smooth Muscle Actin or Desmine are positive. MPNST is of neural origin, so the spindle cells are more wave-shaped, and one end of the nuclei is bulged, and S-100 is positive but epithelial markers negative. Hemangiopericytomas also need to be differentiated from synovial sarcoma. These tumors have vascular changes in all of the tumor area. Cells are polygonal in shape, CD34 positive, and negative for epithelial markers (22).
Treatment of choice of synovial sarcoma of the chest wall as in all soft tissue sarcomas is multimodal combination of wide-to radical resection, radiation therapy and adjuvant chemotherapy following resection and since synovial sarcoma is known to recur, a careful follow up is mandatory (8-10).
Synovial sarcomas might metastasize to bone, liver, skin, the central nervous system, and even breast tissue (23,24).
Prognosis is related to the disease stage and is usually poor. Young age, Her-2 expression, complete resection with clear surgical margins and response to first line chemotherapy were found as good prognostic indicators in advanced disease in different studies (2,25,26). In the other hand, adverse prognostic factors for synovial sarcoma include male gender, truncal as opposed to distal tumor location, lesions larger than 5 cm, high histologic grade (based on the mitotic rate and tumor necrosis), neurovascular invasion, aneuploidy, poor histological differentiation and local recurrence (3,27-30).
Conclusions
Synovial sarcoma is a mesenchymal spindle-cell tumor characterized by variable epithelial differentiation. Its chest wall localization represents a diagnostic challenge because of the diverse array of competing diagnosis and rarity of incidence. Owing to its rarity and the paucity of data regarding its natural history, there are no guidelines for optimal treatment. Meanwhile, it consists on surgical resection associated to chemotherapy and/or radiotherapy. The prognosis remains bleak.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ruggiero A. Synovial sarcoma. Orphanet Encyclopedia. March 2004.
- Spurrell EL, Fisher C, Thomas JM, et al. Prognostic factors in advanced synovial sarcoma: an analysis of 104 patients treated at the Royal Marsden Hospital. Ann Oncol 2005;16:437-44.
- Marzano L, Failoni S, Gallazzi M, et al. The role of diagnostic imaging in synovial sarcoma. Our experience. Radiol Med 2004;107:533-40.
- Bégueret H, Galateau-Salle F, Guillou L, et al. Primary intrathoracic synovial sarcoma: a clinicopathologic study of 40 t(X;18)-positive cases from the French Sarcoma Group and the Mesopath Group. Am J Surg Pathol 2005;29:339-46.
- Miettinen M. Soft tissue tumors with epithelial differentiation. In: Miettinen M. eds. Diagnostic soft tissue pathology. Philadelphia, Pa: Churchill Livingstone, 2003;463-8.
- Satoh H, Ohara G, Hizawa N. Primary synovial sarcoma of the chest wall. J Thorac Oncol 2007;2:1060; author reply 1060.
- Eisenberg RB, Horn RC. Synovial sarcoma of the chest wall; report of a case. Ann Surg 1950;131:281-6, illust.
- Bui-Mansfield LT, Kaplan KJ, Boardman J. Radiologic-pathologic conference of Keller Army Community Hospital at West Point, the United States Military Academy: synovial sarcoma of the chest wall. AJR Am J Roentgenol 2002;179:880.
- Ouadnouni Y, Smahi M, Bouchikh M, et al. A rare tumor of the chest wall: the synovial sarcoma. Pan Afr Med J 2011;9:2.
- Fatimi SH, Saleem T. Giant synovial cell sarcoma of the thorax in a 46-year-old man: a case report. Cases J 2009;2:9324.
- Fujimoto K, Hashimoto S, Abe T, et al. Synovial sarcoma arising from the chest wall: MR imaging findings. Radiat Med 1997;15:411-4.
- Duran-Mendicuti A, Costello P, Vargas SO. Primary synovial sarcoma of the chest: radiographic and clinicopathologic correlation. J Thorac Imaging 2003;18:87-93.
- Fekih L, Boussoffara L, Fenniche S, et al. Rare primary chest wall sarcoma: the synovialosarcoma. Rev Mal Respir 2011;28:681-5.
- Hung JJ, Chou TY, Sun CH, et al. Primary synovial sarcoma of the posterior chest wall. Ann Thorac Surg 2008;85:2120-2.
- Kawano D, Yoshino I, Shoji F, et al. Synovial sarcoma of the chest wall. Gen Thorac Cardiovasc Surg 2010;58:95-7.
- Afif H, El Khattabi W, Maarif H, et al. A thoracic tumor. Rev Med Interne 2006;27:342-3.
- Frazier AA, Franks TJ, Pugatch RD, et al. From the archives of the AFIP: Pleuropulmonary synovial sarcoma. Radiographics 2006;26:923-40.
- Suster S, Moran CA. Primary synovial sarcomas of the mediastinum: a clinicopathologic, immunohistochemical, and ultrastructural study of 15 cases. Am J Surg Pathol 2005;29:569-78.
- Gaertner E, Zeren EH, Fleming MV, et al. Biphasic synovial sarcomas arising in the pleural cavity. A clinicopathologic study of five cases. Am J Surg Pathol 1996;20:36-45.
- Synovial sarcoma. In: Enzinger FM, Weiss SW. eds. Soft tissue sarcomas. St. Louis: Mosby, 1995:757-86.
- Pelmus M, Guillou L, Hostein I, et al. Monophasic fibrous and poorly differentiated synovial sarcoma: immunohistochemical reassessment of 60 t(X;18)(SYT-SSX)-positive cases. Am J Surg Pathol 2002;26:1434-40.
- Yang L, Song B, Lin Z, et al. Clinical pathological analysis of synovial sarcoma. Chin J Clin Oncol 2007;4:246-9.
- Banerjee D, Gorse SJ, Cotter M, et al. Sonographic and pathologic features of metastatic synovial sarcoma of the lung presenting as a breast neoplasm. Breast J 2004;10:372.
- Zeren H, Moran CA, Suster S, et al. Primary pulmonary sarcomas with features of monophasic synovial sarcoma: a clinicopathological, immunohistochemical, and ultrastructural study of 25 cases. Hum Pathol 1995;26:474-80.
- Sápi Z, Pápai Z, Hruska A, et al. Her-2 oncogene amplification, chromosome 17 and DNA ploidy status in synovial sarcoma. Pathol Oncol Res 2005;11:133-8.
- Régnard JF, Icard P, Guibert L, et al. Prognostic factors and results after surgical treatment of primary sarcomas of the lung. Ann Thorac Surg 1999;68:227-31.
- Deshmukh R, Mankin HJ, Singer S. Synovial sarcoma: the importance of size and location for survival. Clin Orthop Relat Res 2004;(419):155-61.
- Bergh P, Meis-Kindblom JM, Gherlinzoni F, et al. Synovial sarcoma: identification of low and high risk groups. Cancer 1999;85:2596-607.
- el-Naggar AK, Ayala AG, Abdul-Karim FW, et al. Synovial sarcoma. A DNA flow cytometric study. Cancer 1990;65:2295-300.
- Trassard M, Le Doussal V, Hacène K, et al. Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol 2001;19:525-34.


