A case of pulmonary arteriovenous malformation: role of interventional radiology in diagnosis and treatment
Introduction
Pulmonary arterio-venous malformations (PAVMs) are abnormal pulmonary arteries and pulmonary veins communicating directly without interposition of a capillary bed. About 80–90% of patients with PAVMs eventually may present with Hereditary Hemorrhagic Telangiectasia (HHT), while others are sporadic. Approximately, 15–35% of HHT patients may present with PAVMs. PAVMs have a tendency to grow and increase in size over time and various factors like puberty, pregnancy and pulmonary arterial hypertension (PAH) affect growth. Early diagnosis, aggressive management and vigilant follow up. This article discusses various manifestations of pulmonary AVMs and discuss in detail the diagnostic features, complications, therapeutic options as well as follow up.
Case presentation
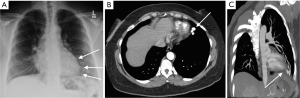
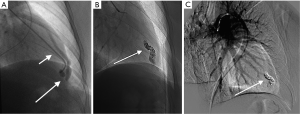
We describe a case of young female presenting with complaints of neurological deficits due to cerebral ischemia with work up revealing PAVMs. The chest X-ray (CXR) revealed a tubular lesion in left lower lobe (Figure 1A). Contrast enhanced computed tomography (CT) angiography of chest showed the presence of feeding artery and draining vein suggestive of AV malformation (Figure 1B,C). Subsequently, the patient underwent successful percutaneous embolization of the PAVM (Figure 2).
Discussion
About 80–90% of patients with PAVMs eventually may present with HHT, remaining ones are sporadic cases. On the other hand, about 15–35% of HHT patients may present with PAVMs (1). The male to female ration for PAVMs is 1.5–1.8:1 (2). About 10% of cases of PAVMs are detected in childhood with gradual increase in detection rates in 5th and 6th decades. The other major causes of PAVMs apart from congenital lesions are trauma, malignancy, hepatopulmonary syndrome in cirrhosis and surgery.
The PAVMs increase in size over time, with factors like puberty, pregnancy and PAH hastening the process. Most PAVMs are unilateral and can affect any part of the lung, but most commonly involving the lower lobes (3). The hereditary forms associated with HHT are often multiple, bilateral and located at lung bases. Secondary PAVMs are found in chest trauma, post-surgical, cirrhosis of liver, mitral stenosis, fungal infections, metastasis and sarcoidosis (4). For diagnosis of HHT, four criteria should be met – namely epistaxis, telangiectasia, visceral lesions and family history. If less than two criteria are met, then HHT is unlikely, but children of affected individual should be considered at higher risk as there is age related penetration in HHT (5). Our patient didn’t meet the criteria for HHT.
Anatomically the PAVMs consist of one or more afferent feeding artery, aneurysmal sac and one or more efferent draining veins. The aneurysmal part of the sac may be either a sac or a bunch of vessels. They can be simple or complex or diffuse. Majority (80%) are simple and contain one or more afferent feeding arteries mostly originating from single segmental pulmonary artery while a complex PAVM (20%) have multiple afferent feeding arteries originating from several segmental arteries, and a diffuse PAVM (5%) consists of hundreds of AVMs which are combination of simple and complex (6,7). Embryological classification consists of five groups, with Group I containing multiple small AVMs without aneurysm, group II containing large AVMs, group III containing large or multiple small AVMs with anomalous venous drainage, group IV containing large venous aneurysm with systemic arterial communication or without fistula and Group V containing anomalous venous drainage with fistulae (4). The developmental process comprises three steps starting with a ground glass nodule representing initial enlargement of the post capillary venules with inflammatory cell infiltrate, then small vessels are visible representing vascular branching and precapillary pulmonary artery and post capillary venules connections and lastly enlargement of the draining vein and disappearance of the ground glass pattern (8).
The PAVMs can be totally asymptomatic or present with cyanosis, congestive heart failure or respiratory failure. The most common presenting symptom is epistaxis due to mucosal telangiectasia. The second common complaint is dyspnea (commonly in patients with large or multiple PAVMs), and third most common symptom being hemoptysis (2). Untreated PAVMs can lead to complications like stroke, abscess, thrombosis, rupture causing hemoptysis or hemothorax or air embolism. The neurological manifestations like cerebrovascular accidents, cerebral abscesses and seizures are due to paradoxical embolism from right to left shunting (9). The number of PAVMs is directly related to prevalence of cortical infarction, being 14% in patients with solitary PAVM to 27% in patients with multiple PAVMs. When all types of cerebral infarctions were considered, the prevalence of infarction increased from 32% in patients with single PAVMs to 60% in patients with multiple PAVMs. However, in another series, 46% of patients with single PAVMs and 59% of patients with multiple PAVMs and almost 70% of patients with diffuse PAVMs had a history of cerebral ischemia or abscess (9). One study concluded that the neurological morbidity related to brain abscess or infarction increases to about 70% in untreated diffuse forms of PAVMs (10).
The diagnosis of PAVMs can be performed with various modalities like pulmonary function tests (PFT), CXR, transthoracic contrast echocardiography (TTCE), radionucleotide lung perfusion scanning, CT scan and 3D MR angiography. The CXR is the initial primary modality in evaluating PAVMs and 98% of patients show some abnormality, classically a lobulated well demarcated mass, mostly in lower lobes (11,12). In PFT, reduction in oxygen saturation has been observed in patients with PAVMs. In a study by Bosher et al., arterial oxygen saturations were less than 76% in 32% of cases, 76–85% in 23% cases, 86–90% in 14% cases, 91–95% in 14% cases and 96–100% in 6% cases (13). TTCE is a simple and minimally invasive examination for screening PAVMs with a diagnostic sensitivity up to 97% and a negative predictive value of 99% (14). It is very helpful in evaluating right to left shunts be it cardiac or intrapulmonary; however, there are limitations due to availability, cost and detection of clinically unimportant shunts (15). One study showed that TTCE can remain positive after successful embolization in a significant number of patients even after 4 months when there was no flow detected on angiography which could be due to small PAVMs not detected by angiography or reperfusion or neo-vascularization through an accessory vessel (16). CT scan is the gold standard in diagnosing PAVMs with advantages of fast scanning, high resolution, characterization, planning interventions and follow up (17). Three-dimensional contrast MR angiography is occasionally used to image thoracic vascular structures (18).
Once diagnosed the treatment should be started early to prevent development of complications secondary to paradoxical embolization, pleuro-pulmonary hemorrhage or hypoxemia. The complication rates increase with increased duration and during pregnancy (19). When the diameter of feeding artery is more than 3 mm, the risk of paradoxical embolization increases; however, few researchers have suggested that even smaller diameter arteries should be embolized early to prevent future growth of PAVMs (20). However, another study reported that in patients with patent foramen ovale, the stroke recurrence rates are unaltered, regardless of the diameter of feeding artery (21).
The treatment options include percutaneous image guided embolization, surgical removal or hormonal therapy. The percutaneous image guided embolization is preferable in most cases with advantages of avoiding general anesthesia and major surgery. The percutaneous transcatheter embolization is used to occlude all PAVMs by catherization of pulmonary arteries selectively. The length of feeding artery decides the complexity of the procedure. Many embolic devices are available like fibered steel coils, fibered platinum coils, hydrophilic coils and self-expandable nitinol plugs. According to Mager’s study, the transcatheter embolizations are successful in majority of patients (83%) (22). In experienced hands, the complications of the procedure are rare, but can include device migration, air embolism, lung infarction or hemoptysis and long-term complications include revascularization or neovascularization (23). In some cases, where the PAVM is supplied by the bronchial artery, there may be delayed hemoptysis due to post-embolic systemic supply. Surgical intervention for PAVMs is aimed at removing the lobe, segment, wedge resection, vascular ligation or pneumonectomy (2). The diffuse forms have required lung transplantation. In patients having PAVMs with symptomatic HHT, the hormonal therapy with systemic estrogen-progesterone at does used for oral contraceptives have been used. This may have additional benefits in women of reproductive age group. Some researchers have reported that anti-hormonal therapy with Tamoxifen has been used to control bleeding from mucosal telangiectasia in HHT (23). Patients with PAVMs should be on anticoagulation therapy. Prophylactic antibiotic therapy is recommended in patients with PAVMs undergoing surgical or dental procedures.
Patients with PAVMs who underwent embolization require follow up at one month and one year after embolization. Some researchers recommend long term follow-up every five years to follow growth of small or new PAVMs to prevent complications from paradoxical embolization (24).
Conclusions
Stroke in the young patients always raises suspicion of some organic cause, requiring aggressive evaluation and appropriate treatment. The diagnostic modalities to investigate such cases must include CXR, echocardiography (transthoracic/transesophageal) with micro-bubble contrast study, CT angiography & magnetic resonance angiography. On establishing the diagnosis of PAVMs, the potential complications should be kept in mind which may be presenting symptoms like stroke, abscess, thrombosis, rupture, hemoptysis/hemothorax, and air embolism. The treatment options include percutaneous transcatheter embolization, surgical removal of lobe or segment, hormonal therapy (estrogen-progesterone at doses used for oral contraception as an initial therapeutic option) and anti-hormonal therapy (tamoxifen) in a few cases. Patients should be kept on anticoagulation therapy and need antibiotic prophylaxis during certain invasive procedures (like dental manipulations). Regular long-term follow-up should be done even after embolization (may be lifelong). As the PAVMs grow over time, patients with treated PAVMs need long-term follow-up every 5 years to detect growth of small PAVMs that may reach a size to cause paradoxical embolization and stroke.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Burke CM, Safai C, Nelson DP, et al. Pulmonary arteriovenous malformations: a critical update. Am Rev Respir Dis 1986;134:334-9. [PubMed]
- Allen SW, Whitfield JM, Clarke DR, et al. Pulmonary arteriovenous malformation in the newborn: a familial case. Pediatr Cardiol 1993;14:58-61. [PubMed]
- Lee EY, Boiselle PM, Cleveland RH. Multidetector CT evaluation of congenital lung anomalies. Radiology 2008;247:632-48. [Crossref] [PubMed]
- Prager RL, Law KH, Bender HW Jr. Arteriovenous fistula of the lung. Ann Thorac Surg 1983;36:231-9. [Crossref] [PubMed]
- Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000;91:66-7. [Crossref] [PubMed]
- Pelage J, Lagrange C, Chinet T, et al. Embolization of localized pulmonary arteriovenous malformations in adults. J Radiol 2007;88:367-76. [Crossref] [PubMed]
- Meek ME, Meek JC, Beheshti MV. Management of pulmonary arteriovenous malformations. Semin Intervent Radiol 2011;28:24-31. [Crossref] [PubMed]
- Lacout A, Marcy PY, Thariat J, et al. VEGF target in HHT lung patients: the role of bevacizumab as a possible alternative to embolization. Med Hypotheses 2012;78:689-90. [Crossref] [PubMed]
- Moussouttas M, Fayad P, Rosenblatt M, et al. White Pulmonary arteriovenous malformations: Cerebral ischemia and neurologic manifestations. Neurology 2000;55:959-64. [Crossref] [PubMed]
- Faughnan ME, Lui YW, Wirth JA, et al. Diffuse pulmonary arteriovenous malformations: characteristics and prognosis. Chest 2000;117:31-8. [Crossref] [PubMed]
- Dines DE, Seward JB, Bernatz PE. Pulmonary arteriovenous fistula. Mayo Clin Proc 1983;58:176-81. [PubMed]
- Motiwala H, Bansal I, Goyal P, et al. Do we really care about incidental lung nodules?-Review of atypical lung carcinoid and a proposal for systematic patient follow up. Transl Lung Cancer Res 2017;6:387-92. [Crossref] [PubMed]
- Bosher LH Jr, Blake DA, Byrd BR. An analysis of the pathologic anatomy of pulmonary arteriovenous aneurysms with particular reference to the applicability of local excision. Surgery 1959;45:91-104. [PubMed]
- Parra JA, Bueno J, Zarauza J, et al. Graded contrast echocardiography in pulmonary arteriovenous malformations. Eur Respir J 2010;35:1279-85. [Crossref] [PubMed]
- Barzilai B, Waggoner AD, Spessert C. Two-dimensional contrast echocardiography in the detection and follow-up of congenital pulmonary arteriovenous malformations. Am J Cardiol 1991;68:1507-10. [Crossref] [PubMed]
- Lee WL, Graham AF, Pugash RA, et al. Contrast echocardiography remains positive after treatment of pulmonary arteriovenous malformations. Chest 2003;123:351-8. [Crossref] [PubMed]
- Remy J, Remy-Jardin M, Giraud F, et al. Angioarchitecture of pulmonary arteriovenous malformations: clinical utility of three-dimensional helical CT. Radiology 1994;191:657-64. [Crossref] [PubMed]
- Maki DD, Siegelman ES, Roberts DA, et al. Pulmonary arteriovenous malformations: three-dimensional gadolinium-enhanced MR angiography-initial experience. Radiology 2001;219:243-6. [Crossref] [PubMed]
- Pierucci P, Murphy J, Henderson KJ, et al. New definition and natural history of patients with diffuse pulmonary arteriovenous malformations: twenty-seven-year experience. Chest 2008;133:653-61. [Crossref] [PubMed]
- Mager JJ, Overtoom TT, Blauw H, et al. Embolotherapy of pulmonary arteriovenous malformations; long-term results in 112 patients. J Vasc Interv Radiol 2004;15:451-6. [Crossref] [PubMed]
- Homma S, Sacco RL, Di Tullio MR, et al. Effect of Medical Treatment in Stroke Patients with Patent Foramen Ovale Patent Foramen Ovale in Cryptogenic Stroke Study. Circulation 2002;105:2625-31. [Crossref] [PubMed]
- Pollak JS, Saluja S, Thabet A, et al. Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations J Vasc Interv Radiol 2006;17:35-44. [Crossref] [PubMed]
- Jameson JJ, Cave DR. Hormonal and antihormonal therapy for epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope 2004;114:705-9. [Crossref] [PubMed]
- White RI Jr, Pollak JS, Wirth JA. Pulmonary arteriovenous malformations: diagnosis and transcatheter embolotherapy. J Vasc Interv Radiol 1996;7:787-804. [Crossref] [PubMed]


