Malignant pleural mesothelioma: adjuvant therapy with radiation therapy
Introduction
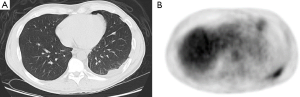
One of the greatest challenges after surgery in malignant pleural mesothelioma (MPM) is local control in the ipsilateral pleura (Figure 1). Radiation therapy (RT) is used in a variety of cancers as an adjuvant treatment with the aim of decreasing the rate of local failure. Post-operative radiation therapy (PORT) is utilized in non-small cell lung cancer (NSCLC), to reduce the risk of tumor recurring in the mediastinum and perhaps improve survival (1). The area at risk in MPM is the entire pleura, so a large radiation field is required increasing the risk of toxicity.
The surgical procedures that have traditionally been used for malignant pleural mesothelioma are extrapleural pneumonectomy (EPP) and pleurectomy/decortication (P/D). EPP involves en bloc resection of the entire pleura, lung and diaphragm, and ipsilateral half of the pericardium. P/D involves resection of all gross disease while leaving the lung intact. Delivering radiotherapy after EPP is aided by the removal of the lung, although it is still difficult due to the inherent risks of treating a patient with only one lung (2). In fact, part of the reason of the utilization of EPP was to allow for the use of high dose radiotherapy.
The role of RT after surgical resection
No consensus exists with regard to the use of RT as a standard treatment modality in mesothelioma. A retrospective review of 663 patients from three institutions demonstrated an improvement in overall survival with the use of multimodality therapy versus surgery alone (3).
The use of radiotherapy has been questioned by an analysis of the SEER database of 14,228 patients with mesothelioma (4). On multivariable analysis, young age, gender (female), lower stage, and surgical intervention were independent predictors of improved survival. The use of surgery alone was demonstrated improved survival when compared to no treatment. There was no difference is survival when combined surgery and radiation was compared to surgery alone. The adjusted hazard ratio for radiation was 1.14 suggesting radiation doesn’t improve survival in MPM.
However, a subsequent analysis utilizing the National Cancer Database (NCDB) suggested a role for adjuvant RT. Ohri and colleagues reviewed 23,414 patients who were entered in the NCDB database between 2004 and 2013. Of these, 14,090 underwent definitive treatment and only 508 (3.6%) received definitive RT. The use of RT improved the 2-year rate of overall survival from 20% to 34%. The adjusted hazard ratio of using radiation was 0.87 (95% confidence interval 0.70–0.87) suggesting a significant benefit with the use of RT. A propensity scored analysis confirmed this result as well (5). It is important to note that population based studies such as those by SEER or NCDB can be difficult to apply to clinical care especially in a disease such as mesothelioma where there is no standardization for the surgical or radiotherapy procedures.
Radiation after EPP
Prior to the advent of modern technology, adjuvant RT after surgery was delivered using standard “two-dimensional” radiotherapy techniques that included matching anterior and posterior fields with electrons radiation delivered to regions where the pleural surface might have been underdosed. Local failure with this technique has been reported to be above 50% by some centers (6).
There have been many technological advances in RT, with one of the most important being the development of intensity modulated radiation therapy (IMRT) for use in a variety of cancers. IMRT is a highly conformal radiation technique that delivers a higher dose to the tumor target while delivering less dose to normal tissues. The goal of this treatment is more effective, but less toxic treatment than conventional techniques (7).
However, IMRT can also lead to inhomogenous dose distribution and excess low dose radiation to other structures such as the contralateral lung, which can subsequently lead to an increased rate of radiation pneumonitis (RP). Harvard reported that in patients treated after EPP with IMRT, there was a 46% rate of grade 5 (fatal) RP (8). Subsequently, there were concerns about the use of IMRT after pneumonectomy. It appeared that toxicity was associated with the dose of radiation incidentally delivered to the remaining lung (9-11).
In the aftermath of the Harvard report, studies were conducted by various institutions to develop guidelines for IMRT in MPM. One of the primary concerns was the dose of RT to the remaining (contralateral) lung. With conventional radiation techniques, there was a relatively low dose to this lung, so there were very low rates of RP.
M.D. Anderson Cancer Center (MDACC) has one of the largest experiences in the use of IMRT after pneumonectomy (12). A retrospective analysis of 86 patients who underwent IMRT after surgery was reported. There was a rate of symptomatic RP in 11.6% of patients. There were five cases of fatal lung toxicity. There are other, less serious side effects from hemithoracic IMRT including fatigue, nausea and esophagitis, which happen in almost all patients. The two-year rate of local control, distant control and overall survival were 55%, 40% and 32%respectively. Only two patients had isolated local failure and 16% of patients in total had any component of local failure. Distant metastases, which also accounts for disease recurrence in the contralateral lung and below the diaphragm was a common failure pattern, occurring in 51 patients (59%).
Since it is challenging to deliver treatment with IMRT, a group studied whether increased experience with received IMRT following EPP led to better treatment plans (13). They examined 30 patients treated with IMRT at their institution and found that the first fifteen patients had improved target coverage when compared to the second group of fifteen patients. Another metric to assess treatment quality is the dose to the heart and lung. This, too, was also reduced in the second fifteen patients. This implies that as experience increases at an institution, including the physicians, physicists, dosimetrists and therapists, the quality of the treatment also improves.
Another type of IMRT is helical tomotherapy (HT). In this technique, a gantry rotates in a full circle around the patient. At the same time, the couch on which the patient is lying and the parts of the treatment machine that modulate the radiation delivery are also changing, allowing for improved treatment delivery. Also in HT, daily imaging, with CT-like scans, verify that the patient is in the correct treatment position.
A study from France, studied the use of HT after EPP (14). Three different clinical target volumes (CTV) were used. CTV1 encompassed the surgical cavity and was treated to 50–54 Gy. CTV2 was delivered to the regions where there was a positive margin after surgery to a dose of 4–6 Gy. CTV3 were elective nodal areas and a dose of 46 Gy was delivered. The goal of treatment planning was to keep the volume of remaining lung receiving 20 Gy less than 20%. There was a rate of grade 3 RP in 16% of patients including two fatal toxicities (8%). Similar to the MDACC study, local failure was fairly low (13%) and all other patients had distant failure.
Stahel et al. published the results of SAKK 17/04, a multi-centered Phase II randomized trial. In this trial, patients received three cycles of induction cisplatin/pemetrexed chemotherapy (15). Patients then received EPP and were subsequently randomized to either hemithoracic RT or no further treatment. There were 153 patients in the study and 113 underwent surgery. However, only 54 patients went on to randomization. There was no significant difference local-regional progression-free survival and overall survival between the two randomized groups. Although the authors concluded that there is no role for post-operative RT after EPP for mesothelioma, it is more likely that this trial was too underpowered to detect any difference between the groups.
Radiation before EPP
Investigators from Princess Margaret Hospital (PMH) in Toronto, Canada have reported on an innovative technique to combine RT and EPP (16). Patients received 25–30 Gy to the entire hemithorax utilizing IMRT one week before EPP. Patients with pathologically involved mediastinal lymph nodes received adjuvant chemotherapy. Out of 62 patients, there was only one patient who died in the hospital after EPP and two patients who died after discharge for a treatment related mortality of 5%. Twenty-four patients (39%) developed grade 3 or higher toxicity which was mostly atrial fibrillation or empyema. It is important to note that once the RT has been delivered, surgery is obligatory. It can have presumed that if surgery is not performed, there would be significant radiation pneumonitis. In the PMH study, no patient underwent RT without subsequently having surgical resection. The median survival for all patients as an intention-to-treat analysis was an encouraging 36 months. An accompanying editorial suggested that an aggressive approach such as SMART should only be attempted in centers with significant surgical and radiation oncology expertise (17). When this treatment was compared to the use of neoadjuvant chemotherapy, it was found to have similar surgical risk (18).
Radiation after P/D
As the use of P/D has increased in popularity over EPP (3), it became important to develop techniques to treat the pleura and chest wall with the remaining lung in the treatment field. Conventional radiotherapy techniques had been used with limited success. In a large retrospective trial evaluating this treatment the local control rate was 42% at one year and the median overall survival was 13.5 months (19). The treatment technique had to be modified from the post-EPP method to account for the remaining lung and additional blocks were utilized. This compromised the ability to deliver therapeutic doses of RT and might have led to the poor outcomes. Additionally, the treatment had a 28% rate of grade 3–4 toxicity with two patients having fatal toxicity.
With the improvements in RT treatment deliver, institutions began utilizing hemithoracic pleural IMRT (also known as Intensity Modulated Pleural RadIatioN Therapy (IMPRINT)) after P/D (Figure 2). Since the ipsilateral lung is still intact, the clinical situation is somewhat similar to lung cancer and some of the same dosimetric considerations apply.
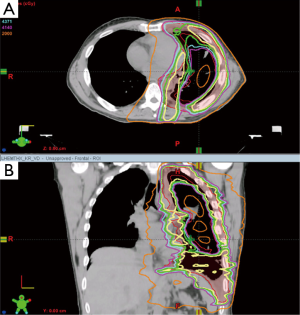
Rosenzweig, et al., from Memorial Sloan-Kettering Cancer Center (MSKCC) reported on the feasibility of pleural IMRT in thirty-six patients treated who had P/D or no surgery (20). The median dose was 4,680 cGy and 90% of the patients had received sequential (not concurrent) chemotherapy. The treatment was delivered via a static field technique (also known as “step and shoot”). Beams typically entered from eight separate angles around the patient. Twenty percent of patients developed grade 3 or worse toxicity, including one toxic death. Five patients (16%) had chronic RP. The conclusion of the study was that pleural IMRT is a feasible and a relatively safe treatment technique for patients with MPM who have an intact ipsilateral lung. Post-treatment imaging of a patient who received IMPRINT is presented in Figure 3. A recent study demonstrates that the use of advanced IMRT techniques, such as VMAT can improve the radiation dose distribution and reduce predicted toxicity (21).
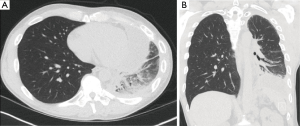
MSKCC updated their experience and reported on patients treated with hemithoracic pleural IMRT (22). The goal of this study was to determine the location of local failure and correlate it with characteristics such as imaging, the treatment plan, and post-treatment imaging. Failures were categorized as in-field, marginal, out-of-field, or distant depending on the failures’ relation to the 90% and 50% isodose lines. The median time to local failure was ten months from the conclusion of pleural IMRT. The median follow-up for all patients in the study was 24 months from diagnosis. The rate of local failure was 56% and 74% and one and two years, respectively. In total, there were 43 (64%) in-field local failures. For patients who underwent P/D versus those who received less extensive surgery, the median time to local failure was 14 months and 6 months, respectively (P<0.03). The authors concluded that local failure is the predominant pattern of failure and that patients treated with hemithoracic pleural IMRT in the adjuvant setting after P/D experience a significant prolongation in time to local and distant failure than patients treated with IMRT after less than a complete resection.
A two-center Phase II study evaluating the use IMPRINT with chemotherapy and P/D was recently reported (23) from MSKCC and MDACC. Twenty-seven patients received radiation after chemotherapy and P/D (median dose, 46.8 Gy). Six patients had grade 2 RP, and two patients developed grade 3 or worse RP (no grade 4 or 5). The median progression-free survival and overall survival (OS) were 12.4 and 23.7 months, respectively. The 2-year OS was 59% in patients with resectable tumors and was 25% in patients with unresectable tumors. A follow-up multi-institutional study of the IMPRINT technique is currently underway.
Another study reported on the use of helical tomotherapy after P/D or biopsy alone (24). A total of 28 patients were treated to an intended dose of 50 Gy. PET scans were obtained on all patients and regions with increased uptake received an additional 10 Gy. The CTV extended from the top of the lung to the below the diaphragm and included involved mediastinal lymph nodes. The CTV was expanded 5mm to create the planning target volume. The primary pulmonary dosimetric constraint was the contralateral lung to a mean dose of less than 7 Gy. The ipsilateral and total lung did not have specific dose constraints. Five patients (18%) had pulmonary toxicity, but only two were grade 3 (7%) and none were grade 5. Contralateral lung V5 was strongly correlated with the risk of pneumonitis. This is provocative considering that there should be some remaining function in the ipsilateral lung.
A comparison of the IMPRINT technique and conventional RT was recently performed (25). Conventional RT consisted of matched photon/electron fields and was delivered with two-dimensional RT techniques. Overall survival was significantly higher after IMPRINT (median 20.2 vs. 12.3 months, P=0.001). Additionally, fewer patients developed grade ≥2 esophagitis after IMPRINT compared to CONV (23% vs. 47%).
Conclusions
Many aspects of treatment for patients with malignant pleural mesothelioma are still not standardized. There is still variation in the surgical technique used and the role of RT. The use of pemetrexed chemotherapy is standard, but there is still no clinically effective second line systemic treatment. Similar to other solid tumors, there has been some promising work with monoclonal antibodies and immunotoxins (26,27).
The use of RT has changed radically with the advent of advanced radiation treatment planning techniques, especially IMRT. IMRT is now part of the care for almost all patients when RT is used, despite the difficulties in some of the earliest studies. Since patients with mesothelioma frequently develop the disease from environmental exposures they typically have other medical comorbidities which can make them a difficult patient population to treat.
Many centers have limited their use of EPP in favor of P/D in an effort to decrease operative toxicity, especially given the possibility that there may not be a significant difference in clinical outcome between the two techniques. Therefore, radiation oncologists will be evaluating patients with two intact lungs in need of adjuvant RT. IMRT, with its ability to deliver concave doses of RT to complex geometries is a logical solution to this problem.
Recent studies in the use of IMRT show that the safety has improved with experience and has excellent efficacy in single institutional reports. Most of these studies are from centers with extensive experience in treating patients with MPM and are able to develop expertise in the disease. The clinical issues for these patients, including tumor contouring, treatment planning and treatment delivery are not inconsiderable. Additionally, although the toxicity for these treatments has been reduced, it is not insignificant and must be taken into consideration when treating our patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Robinson CG, Patel AP, Bradley JD, et al. Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Data Base. J Clin Oncol 2015;33:870-6. [Crossref] [PubMed]
- Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001;122:788-95. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.e1-3.
- Taioli E, Wolf AS, Camacho-Rivera M, et al. Determinants of Survival in Malignant Pleural Mesothelioma: A Surveillance, Epidemiology, and End Results (SEER) Study of 14,228 Patients. PloS One 2015;10:e0145039. [Crossref] [PubMed]
- Ohri N, Taioli E, Ehsani M, et al. Definitive Radiation Therapy Is Associated With Improved Survival in Non-Metastatic Malignant Pleural Mesothelioma. Int J Radiat Oncol Biol Phys 2016;96:S132. [Crossref]
- Yajnik S, Rosenzweig KE, Mychalczak B, et al. Hemithoracic radiation after extrapleural pneumonectomy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2003;56:1319-26. [Crossref] [PubMed]
- Krayenbuehl J, Oertel S, Davis JB, et al. Combined photon and electron three-dimensional conformal versus intensity-modulated radiotherapy with integrated boost for adjuvant treatment of malignant pleural mesothelioma after pleuropneumonectomy. Int J Radiat Oncol Biol Phys 2007;69:1593-9. [Crossref] [PubMed]
- Allen AM, Czerminska M, Janne PA, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys 2006;65:640-5. [Crossref] [PubMed]
- Kristensen CA, Nottrup TJ, Berthelsen AK, et al. Pulmonary toxicity following IMRT after extrapleural pneumonectomy for malignant pleural mesothelioma. Radiother Oncol 2009;92:96-9. [Crossref] [PubMed]
- Miles EF, Larrier NA, Kelsey CR, et al. Intensity-modulated radiotherapy for resected mesothelioma: the Duke experience. Int J Radiat Oncol Biol Phys 2008;71:1143-50. [Crossref] [PubMed]
- Rice DC, Smythe WR, Liao Z, et al. Dose-dependent pulmonary toxicity after postoperative intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2007;69:350-7. [Crossref] [PubMed]
- Gomez DR, Hong DS, Allen PK, et al. Patterns of failure, toxicity, and survival after extrapleural pneumonectomy and hemithoracic intensity-modulated radiation therapy for malignant pleural mesothelioma. J Thorac Oncol 2013;8:238-45. [Crossref] [PubMed]
- Patel PR, Yoo S, Broadwater G, et al. Effect of increasing experience on dosimetric and clinical outcomes in the management of malignant pleural mesothelioma with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2012;83:362-8. [Crossref] [PubMed]
- Giraud P, Sylvestre A, Zefkili S, et al. Helical tomotherapy for resected malignant pleural mesothelioma: dosimetric evaluation and toxicity. Radiother Oncol 2011;101:303-6. [Crossref] [PubMed]
- Stahel RA, Riesterer O, Xyrafas A, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncol 2015;16:1651-8. [Crossref] [PubMed]
- de Perrot M, Feld R, Leighl NB, et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2016;151:468-73. [Crossref] [PubMed]
- Rusch VW, Rimner A, Adusumilli PS. SMART or simply bold? J Thorac Cardiovasc Surg 2016;151:476-7. [Crossref] [PubMed]
- Mordant P, McRae K, Cho J, et al. Impact of induction therapy on postoperative outcome after extrapleural pneumonectomy for malignant pleural mesothelioma: does induction-accelerated hemithoracic radiation increase the surgical risk? Eur J Cardiothorac Surg 2016;50:433-8. [Crossref] [PubMed]
- Gupta V, Mychalczak B, Krug L, et al. Hemithoracic radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2005;63:1045-52. [Crossref] [PubMed]
- Rosenzweig KE, Zauderer MG, Laser B, et al. Pleural intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2012;83:1278-83. [Crossref] [PubMed]
- Kuo L, Yorke ED, Dumane VA, et al. Geometric dose prediction model for hemithoracic intensity-modulated radiation therapy in mesothelioma patients with two intact lungs. J Appl Clin Med Phys 2016;17:371-9. [Crossref] [PubMed]
- Rimner A, Spratt DE, Zauderer MG, et al. Failure patterns after hemithoracic pleural intensity modulated radiation therapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2014;90:394-401. [Crossref] [PubMed]
- Rimner A, Zauderer MG, Gomez DR, et al. Phase II Study of Hemithoracic Intensity-Modulated Pleural Radiation Therapy (IMPRINT) As Part of Lung-Sparing Multimodality Therapy in Patients With Malignant Pleural Mesothelioma. J Clin Oncol 2016;34:2761-8. [Crossref] [PubMed]
- Minatel E, Trovo M, Polesel J, et al. Tomotherapy after pleurectomy/decortication or biopsy for malignant pleural mesothelioma allows the delivery of high dose of radiation in patients with intact lung. J Thorac Oncol 2012;7:1862-6. [Crossref] [PubMed]
- Shaikh F, Zauderer MG, von Reibnitz D, et al. Improved Outcomes with Modern Lung-Sparing Trimodality Therapy in Patients with Malignant Pleural Mesothelioma. J Thorac Oncol 2017;12:993-1000. [Crossref] [PubMed]
- Hassan R, Sharon E, Thomas A, et al. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014;120:3311-9. [Crossref] [PubMed]
- Hassan R, Kindler HL, Jahan T, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res 2014;20:5927-36. [Crossref] [PubMed]


